Antigen protein and nucleic acid coding for said protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Induction of Infection Resistance and Involvement of CD4-Positive T-Cells in Infection Resistance
1) Induction of Infection Resistance
[0108]Candida albicans (C. albicans) TIMM 1768 was cultured overnight with shaking in Sabouraud dextrose medium. Thereafter, cultured cells were harvested by centrifugation and washed with physiological saline. The obtained cells were suspended in physiological saline so as to have concentrations of 1×106, 1×107, and 1×108 cells / ml. An equal volume of IFA (incomplete Freund's adjuvant) was added to each suspension and mixed. This mixture was subcutaneously administered to C57BL / 6 mice at 0.1 ml per animal, thereby immunizing the mice with live Candida cells. After 1 week, the mice were further immunized by subcutaneously administering the same number of live Candida cells prepared in the same manner as above. Therefore, each mouse was twice immunized with 5×104, 5×105, or 5×106 live Candida cells. As a control, a mixture of physiological saline and ...
example 2
Collection of Serum from Mammal Having Infection Resistance, Characteristics Thereof, and Preparation of Various Absorption Sera
1) Collection of Serum from Infection-Resistant Mammal
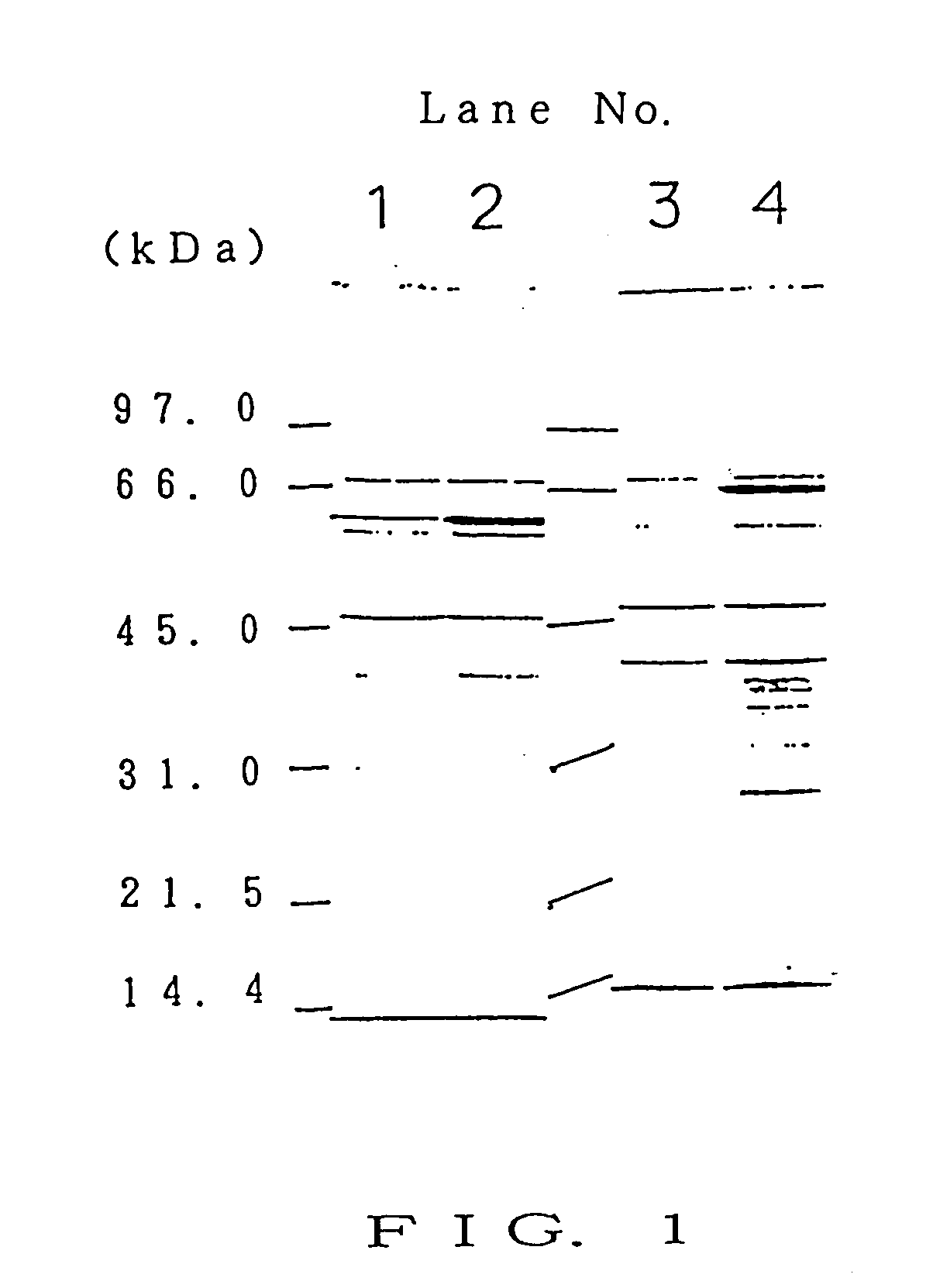
[0114] Serum was collected from BALB / c mice that were immunized 3 times with 5×106 live C. albicans TIMM 1768 cells in a mixture with CFA (complete Freund's adjuvant) in the same manner as in item 2) of Example 1 by a conventional method at 7 days after final immunization. This resulting serum was used as an anti-Candida serum.
2) Characteristics of Serum from Infection-Resistant Mammal
[0115] The characteristics of the anti-Candida serum obtained in item 1) of Example 2 were studied.
[0116] First, as antigenic components, C. albicans cell wall fraction (CW), cytoplasm fraction (HSS), and cell membrane fraction (LSP) were prepared.
[0117] A loopful of C. albicans TIMM 1768 cells in Sabouraud agar slant culture was transferred to a test tube containing 5 ml of YPD medium (1% by weight yeast extract, 2...
example 3
Screening of C. albicans cDNA Expression Library for Antigenic Proteins
1) Preparation of C. albicans cDNA Expression Library
[0123] Total RNA was extracted and purified from cells to prepare a cDNA expression library for C. albicans TIMM 1768 strain. Specifically, the above strain was cultured at 35° C. in 200 ml of YPD medium, and thereafter the cells were harvested by centrifugation (2,000 rpm, 5 minutes) and washed once with distilled water. The cells were quickly frozen with liquid nitrogen, and the frozen cells were disrupted with a mortar into a powdery form. Total RNA was recovered and purified from this cell powder using an RNA extraction kit (manufactured by Pharmacia). poly(A)+ RNA was prepared using Oligotex™-dT30 (manufactured by Takara Shuzo Co., Ltd.) from this total RNA. cDNA was synthesized from 5 μg of the poly(A)+ RNA using cDNA synthesis kit (manufactured by Takara Shuzo Co., Ltd.). A cDNA library was constructed by ligating the synthesized cDNA to lambda phage...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com