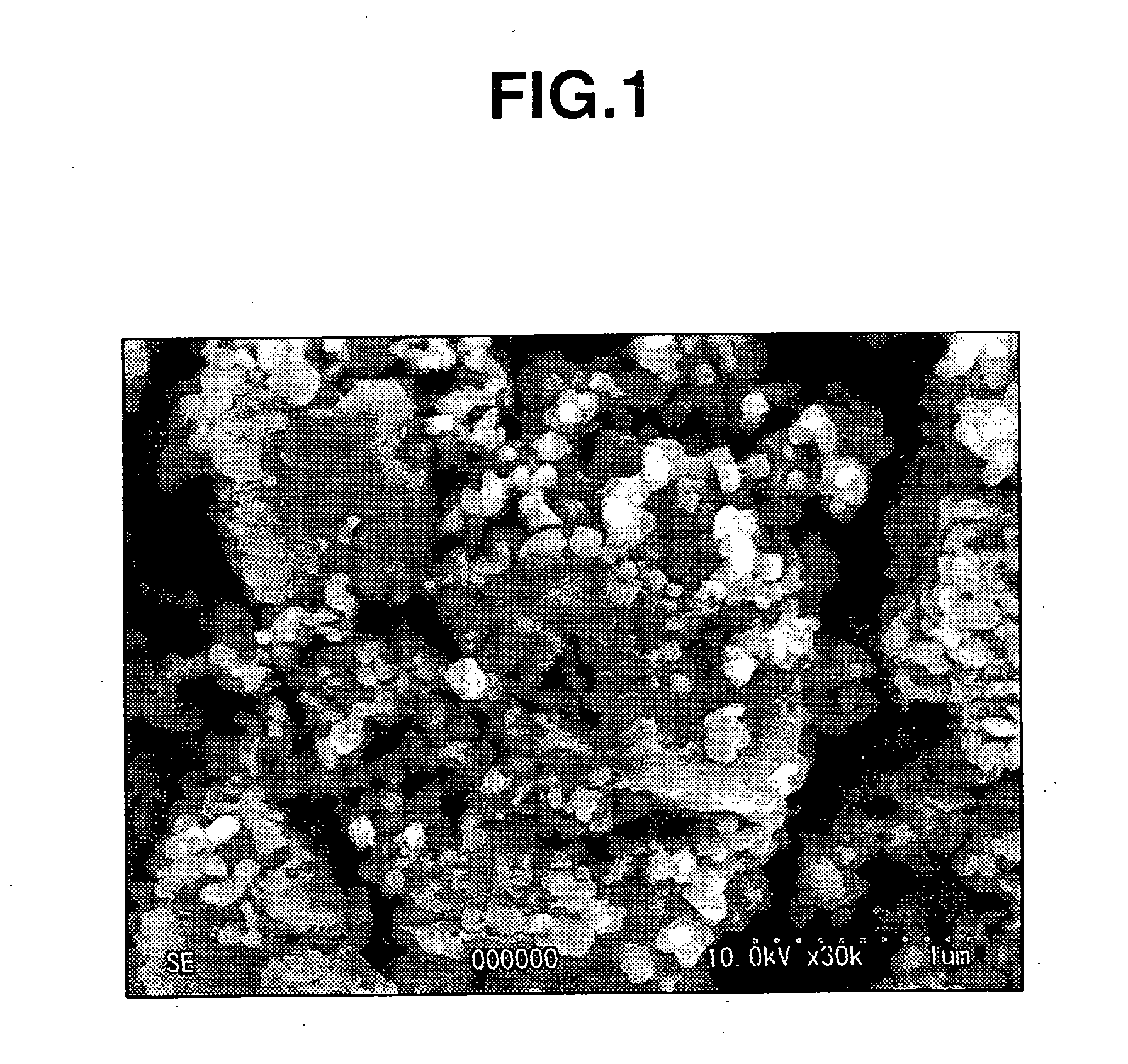
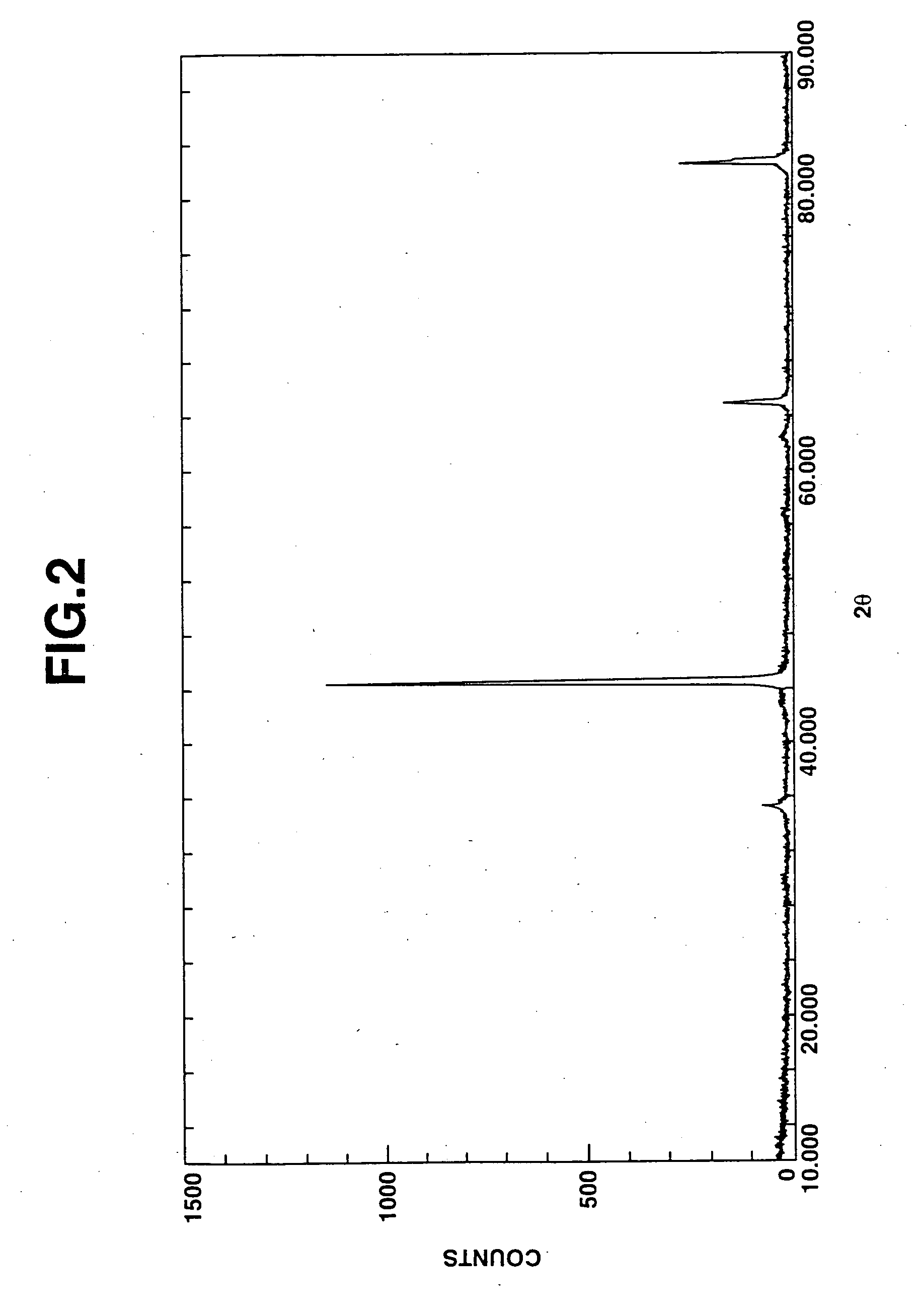
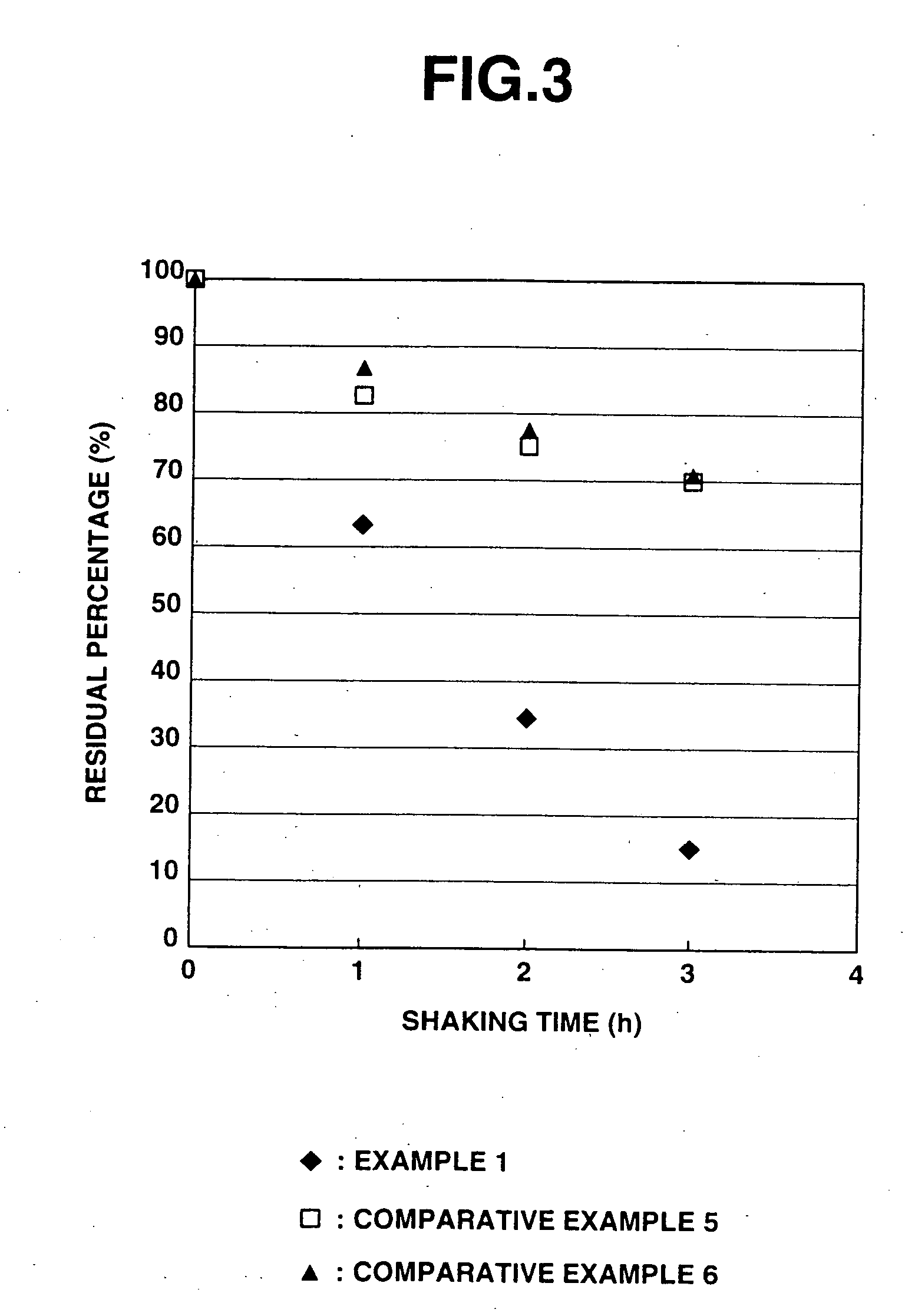
Iron particles for purifying contaminated soil or ground water
a technology of iron particles and soil, which is applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, and separation processes, etc., can solve the problems of ineffective treatment methods of pcb past used, the amount of pcb is extremely high, and the amount of pcb is presently prohibited
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0238] A reaction vessel maintained in a non-oxidative atmosphere by flowing N2 gas at a rate of 3.4 cm / sec, was charged with 704 liters of a 1.16 mol / l Na2CO3 aqueous solution, and then with 296 liters of an aqueous ferrous sulfate solution containing 1.35 mol / l of Fe2+ (amount of Na2CO3: 2.0 equivalents based on equivalent of Fe), and these solutions were mixed together at 47° C., thereby producing FeCO3.
[0239] The aqueous solution containing the thus obtained FeCO3 was successively held at 47° C. for 70 minutes while blowing N2 gas thereinto at a rate of 3.4 cm / sec. Thereafter, air was passed through the FeCO3-containing aqueous solution at a temperature of 47° C. and a flow rate of 2.8 cm / sec for 5.0 hours, thereby producing goethite particles 1. Meanwhile, it was confirmed that the pH value of the aqueous solution during the air passage was maintained at 8.5 to 9.5.
[0240] The water suspension containing the thus obtained goethite particles 1 was washed with water using a filt...
example 2
[0252] A reaction vessel maintained in a non-oxidative atmosphere by flowing N2 gas therethrough at a rate of 3.4 cm / sec, was charged with 704 liters of a 1.16 mol / l Na2CO3 aqueous solution, and then with 296 liters of an aqueous ferrous sulfate solution containing Fe2+ in an amount of 1.35 mol / l (amount of Na2CO3: 2.0 equivalents based on equivalent of Fe), and these solutions were mixed together at 47° C., thereby producing FeCO3.
[0253] The aqueous solution containing the thus obtained FeCO3 was successively held at 47° C. for 70 minutes while blowing N2 gas thereinto at a rate of 3.4 cm / sec. Thereafter, air was passed through the FeCO3-containing aqueous solution at a temperature of 47° C. and a flow rate of 2.8 cm / sec for 5.0 hours, thereby producing goethite particles 4. Meanwhile, it was confirmed that the pH value of the aqueous solution during the air passage was maintained at 8.5 to 9.5.
[0254] The water suspension containing the thus obtained goethite particles 4 was wash...
example 3
[0265] A reaction vessel maintained in a non-oxidative atmosphere by flowing N2 gas therethrough at a rate of 3.4 cm / sec, was charged with 704 liters of a 1.16 mol / l Na2CO3 aqueous solution, and then with 296 liters of an aqueous ferrous sulfate solution containing Fe2+ in an amount of 1.35 mol / l (amount of Na2CO3: 2.0 equivalents based on equivalent of Fe), and these solutions were mixed together at 47° C., thereby producing FeCO3.
[0266] The aqueous solution containing the thus obtained FeCO3 was successively held at 47° C. for 70 minutes while blowing N2 gas thereinto at a rate of 3.4 cm / sec. Thereafter, air was passed through the FeCO3-containing aqueous solution at a temperature of 47° C. and a flow rate of 2.8 cm / sec for 5.0 hours, thereby producing goethite particles 5. Meanwhile, it was confirmed that the pH value of the aqueous solution during the air passage was maintained at 8.5 to 9.5.
[0267] The water suspension containing the thus obtained goethite particles 5 was wash...
PUM
| Property | Measurement | Unit |
|---|---|---|
| BET specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com