Activated carbon monolith catalyst, methods for making same, and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0075] Approximately 2 L of de-ionized water was added to a 3 L heated glass reactor, and agitated by a variable speed motor attached to a plastic impeller. The temperature was ambient, and recorded via a thermocouple connected to a recording device. A quantity of sodium carbonate was added to the water in the stirring reactor so as to elevate the pH to about 10.5.
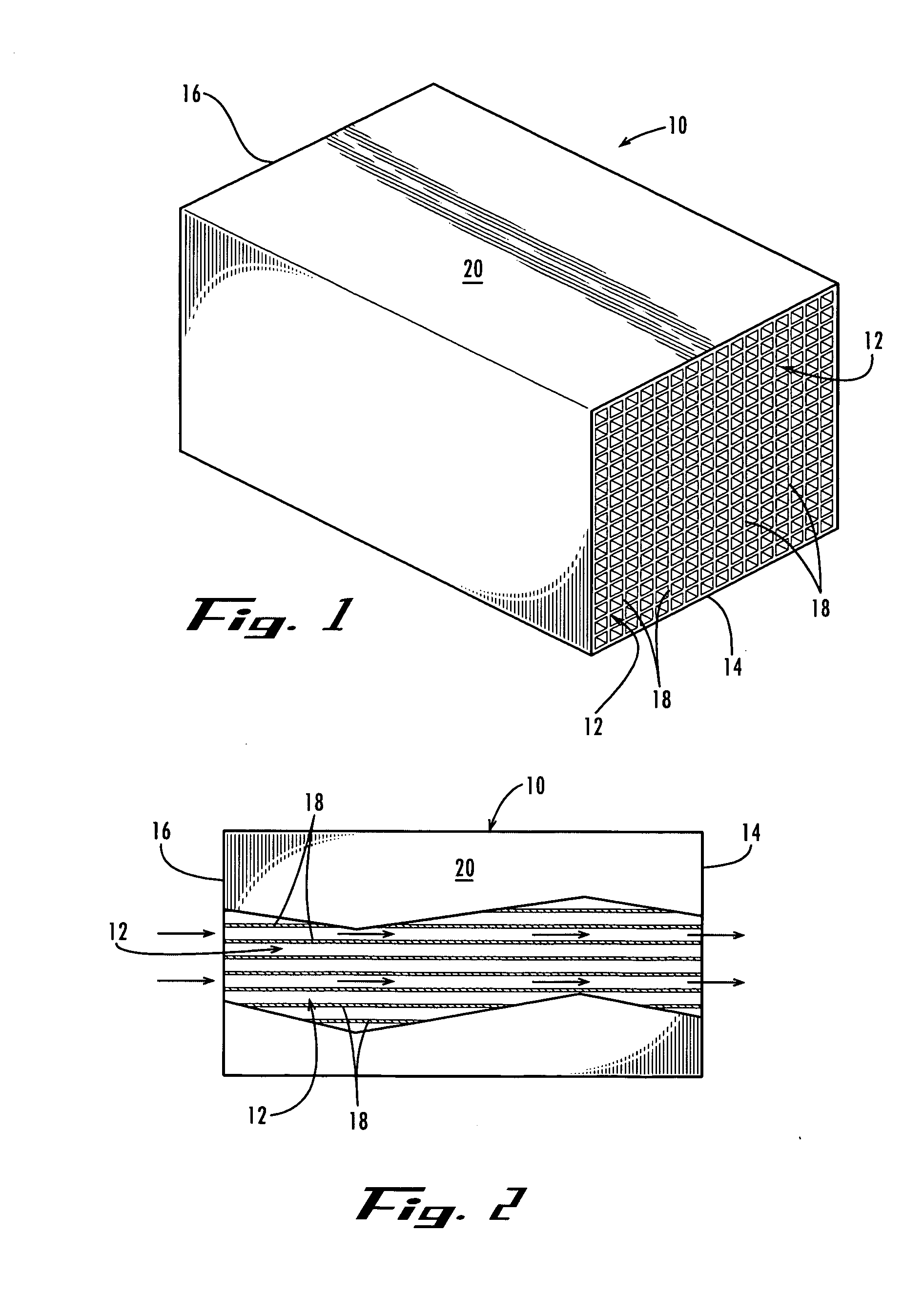
[0076] A finished self-supporting activated carbon monolith made in accordance with U.S. Pat. No. 5,914,294 was placed in the reactor so as to have the sodium carbonate aqueous solution pass evenly through the cells of the monolith as the solution was agitated.
[0077] In another glass container, a solution of palladium chloride was prepared so as to have a palladium metal loading by weight of the carbon monolith of 0.1%. The pH of this solution was adjusted to a pH of 4.0 using sodium bicarbonate. This solution was metered into the reactor.
[0078] After the metering of the palladium solution, the reactor was heated via an...
example 2
[0081] In the same manner of Example 1, a finished self-supporting activated carbon monolith made in accordance with U.S. Pat. No. 5,914,294 was used to prepare a catalyst with a palladium metal loading of 5% by weight of the activated carbon monolith catalyst.
[0082] Ingredients were increased proportionally to the amount of palladium metal used in this Example 2, as compared to Example 1.
example 3
[0083] The activated carbon monolith catalyst of Example 2 was tested for its catalytic activity using nitrobenzene as a test reactant.
[0084] The activated carbon monolith catalyst was placed in the 500 ml glass bottle of a Rocking Parr Bomb. A quantity of 2 ml of pure nitrobenzene was added to the glass bottle along with 50 ml of methanol to act as a solvent. The bottle was inserted into the Rocking Parr Bomb at ambient temperature, which was 22° C. at the time of the test.
[0085] The bottle was pressurized to 60 psig with pure hydrogen. When agitation of the bottle commenced, time and hydrogen pressure in the bottle were recorded. Hydrogen pressure was seen to fall from 60 psig to 43.5 psig in 255 seconds. The temperature of the contents of the bottle were seen to rise from 22° C. to 31° C. in the same time period. These are direct indications of a catalytic reaction occurring with the nitrobenzene and hydrogen due to the presence of the activated carbon monolith catalyst in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com