Mg-Ni hydrogen storage composite having high storage capacity and excellent room temperature kinetics
a hydrogen storage composite and high storage capacity technology, applied in the direction of metal/metal-oxide/metal-hydroxide catalysts, cell components, physical/chemical process catalysts, etc., can solve the problems of high cost of containers, high storage capacity of containers, hazardous and heavy containers, etc., to achieve high total storage capacity, high adsorption rate, and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
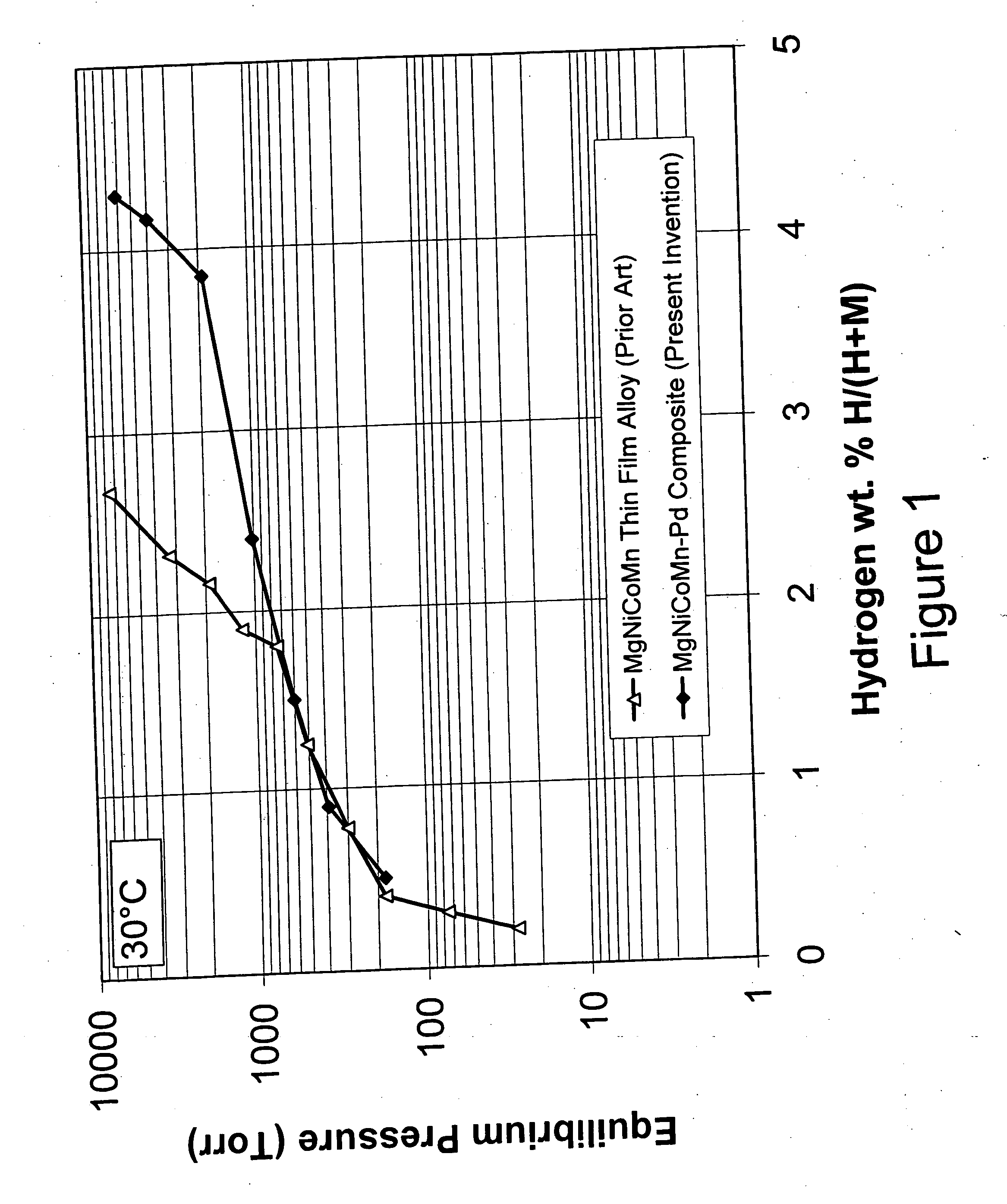
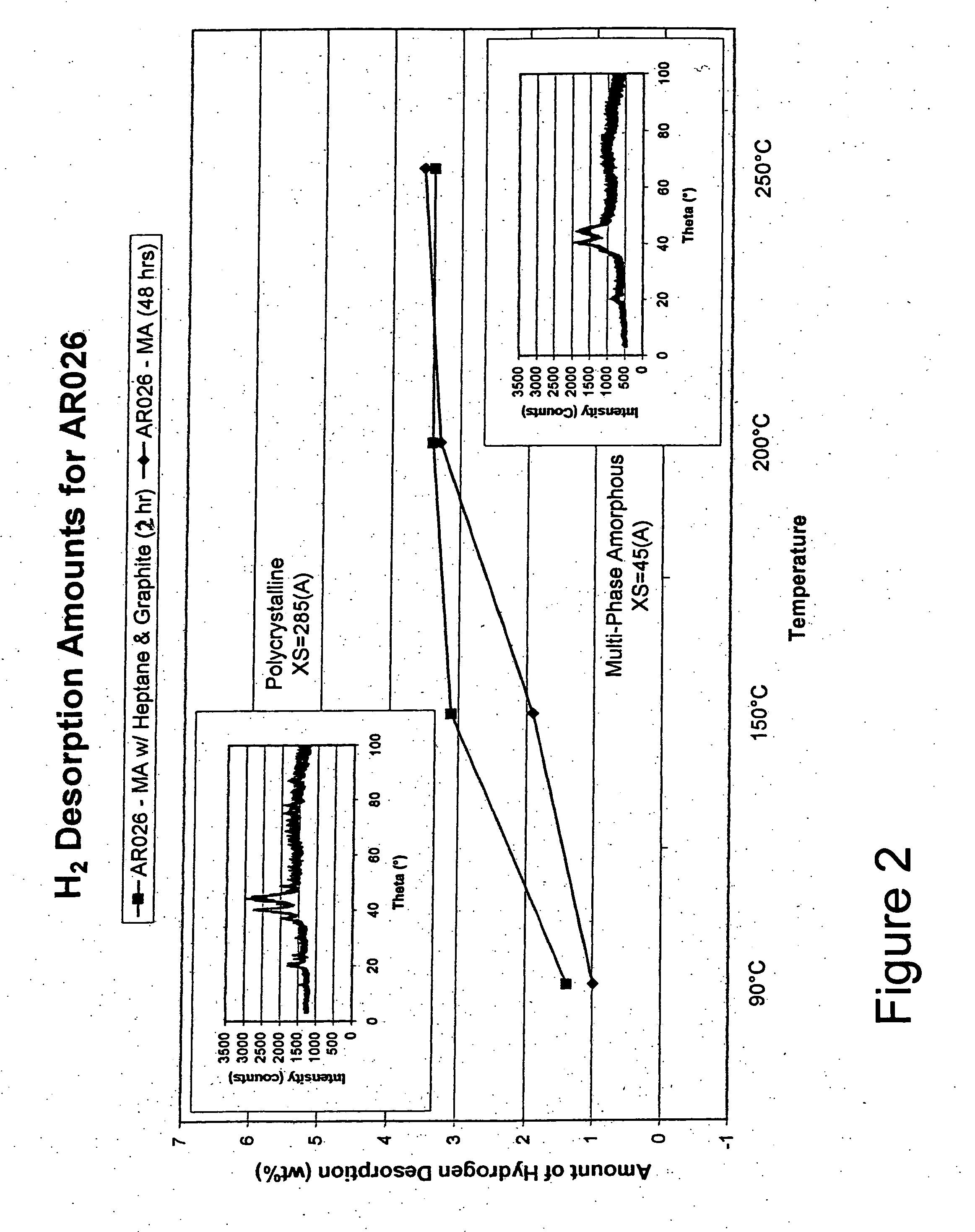
[0080] The Mg—Ni alloy composite materials of the instant invention exhibit, for the first time ever, the ability to store and release significant quantities of hydrogen at temperatures less than about 100° C. with good kinetics. Specifically, the instant composite materials can store greater than about 3 weight percent hydrogen at 30° C. More preferably these materials can store greater than about 3.5 weight percent hydrogen and most preferably they can store more than about 4 weight percent hydrogen at 30° C. The base alloys are produced by melt spinning and mechanical alloying and have an addition of a minute quantity of palladium and / or iron on at least a portion of the surface of the alloy to form the composite. As discussed hereinafter, the conditions of the melt spinning and mechanical alloying of the base alloy play a major role in creating the unique properties of the instant composite materials.
[0081] The preferred composite materials of the instant invention generally co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com