Recombinant alpha-L-iduronidase, methods for producing and purifying the same and methods for treating diseases caused by deficiencies thereof
a technology of recombinant alpha-l-iduronidase and purification method, which is applied in the field of recombinant alpha-l-iduronidase, can solve the problems of high morbidity and mortality, low production level, and low clinical efficacy, and achieves preservation or enhancement of the biological activity of the enzyme and high production level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Producing Recombinant Iduronidase
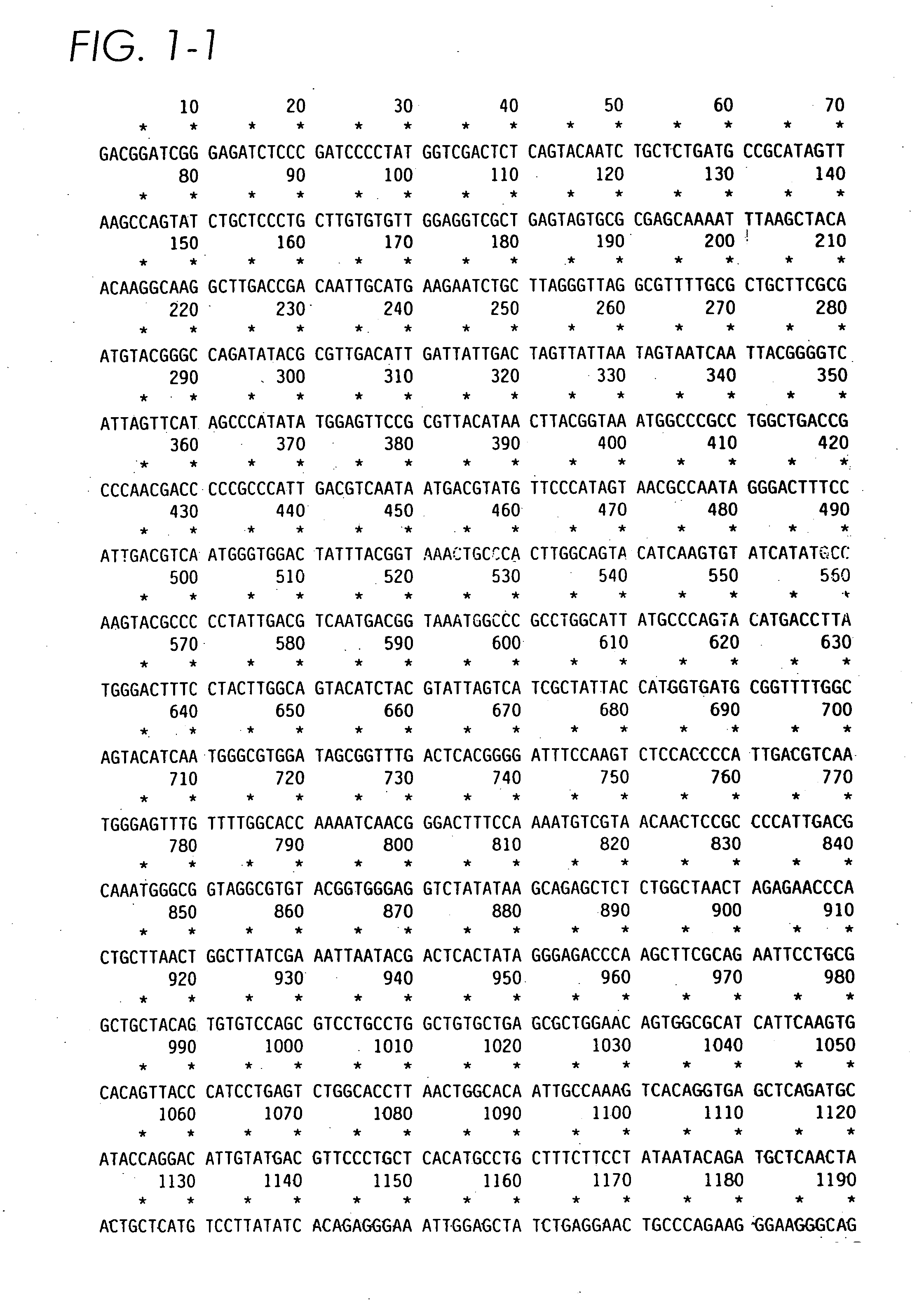
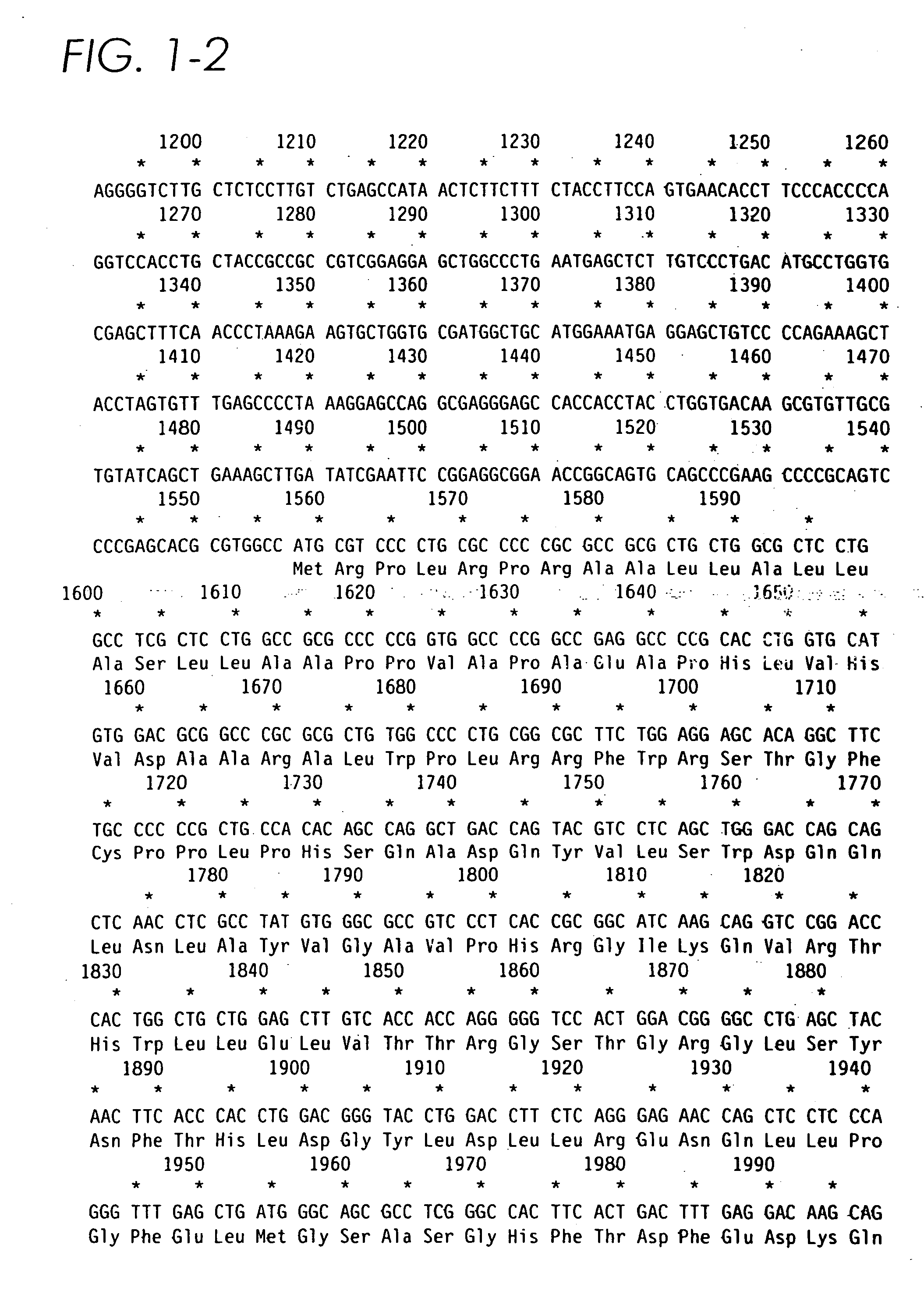
[0072] Standard techniques such as those described by Sambrook et al. (1987) “Molecular Cloning: A Laboratory Manual”, 2nd ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. may be used to clone cDNA encoding human α-L-iduronidase. The human α-L-iduronidase cDNA previously cloned was subcloned into PRCCMV (InVitrogen) as a HindIII-XbaI fragment from a bluescript KS subclone. An intron cassette derived from the murine immunoglobulin Cot intron between exons 2 and 3 was constructed using PCR amplification of bases 788-1372 (Tucker et al., Proc. Natl. Acad. Sci. USA 78: 7684-7688 (1991) of clone pRIR14.5 (Kakkis et al., Nucleic Acids Res. 16:7796 (1988)). The cassette included 136 bp of the 3′ end of exon 2 and 242 bp of the 5′ end of exon 3, which would remain in the properly spliced cDNA. No ATG sequences are present in the coding, region of the intron cassette. The intron cassette was cloned into the HindIII site 5′ of the α-L-iduronidase ...
example 2
[0077] Seed train: A vial of working cell bank (WCB) CHO cells 2.131 is partially thawed in a 37° C. water bath. The thawed cells are added to seven mL of fresh cell culture medium (DMEM / F12) containing thymidine (7.6 mg / L), hypoxanthine (13.6 mg / L), G418 (375 mg / mL) and fetal bovine serum (5%). The cells are collected by gentle centrifugation (400 r.p.m. for 5 minutes). The pelleted cells are resuspended in fresh DMEM / F12 medium substituted as described above and the cells are placed in a T-75 cm flask in 25 mL of the medium.
[0078] After 2 to 3 days of incubation at 37° C. and 5% carbon dioxide, the cells are placed in a T-225 flask with 50 mL of fresh medium. The next split of cells is into a 250 mL spinner (100 mL of cells and medium) and the agitator (spinner) is rotated at 50 r.p.m. The inoculum volume and cell number is increased by a series of incubations (cell density increases) and subculturing (cell volume increases). The subculturing is performed such that the final cell...
example 3
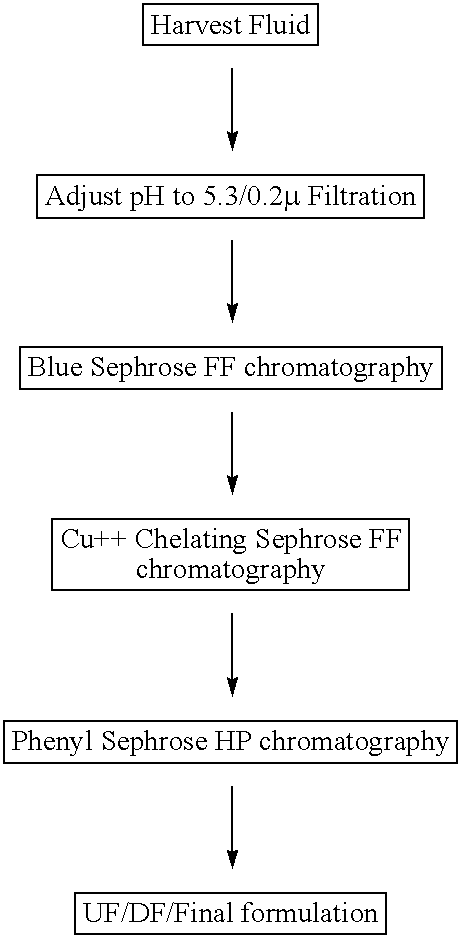
[0083] Recombinant iduronidase over-expressed in a Chinese Hamster Ovary (CHO) cell line, has been purified to near homogeneity following a 3 step column chromatography process. The first column involves an affinity chromatography step using Blue Sepharose 6 FF. The Blue eluate is then further purified by another affinity chromatography step using Cu++ Chelating Sepharose FF. The final polish of the highly purified enzyme is achieved by hydrophobic interaction chromatography using Phenyl Sepharose High Performance (HP). The over-all yield ranges from 45 to 55 percent and the purity of the final product is >99%. The process is robust, reproducible, and scalable for large-scale manufacturing. The purified enzyme has been characterized with respect to its enzymatic activity using a fluorescence-based substrate, and its functional uptake by fibroblast cells. The enzyme has also been characterized for substrate specificity, carbohydrate profiles, and isoelectric focusing (IEF) profiles. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com