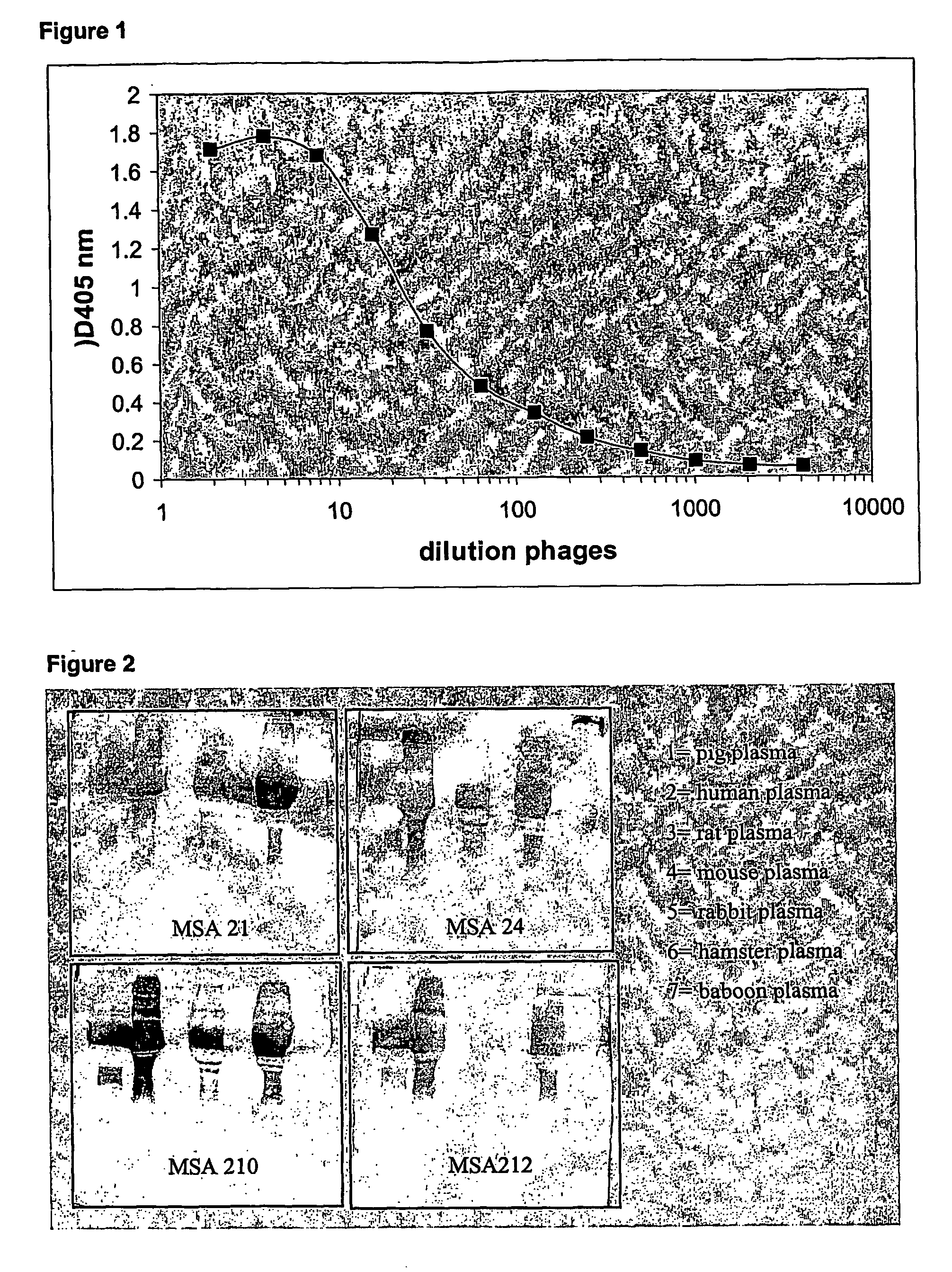
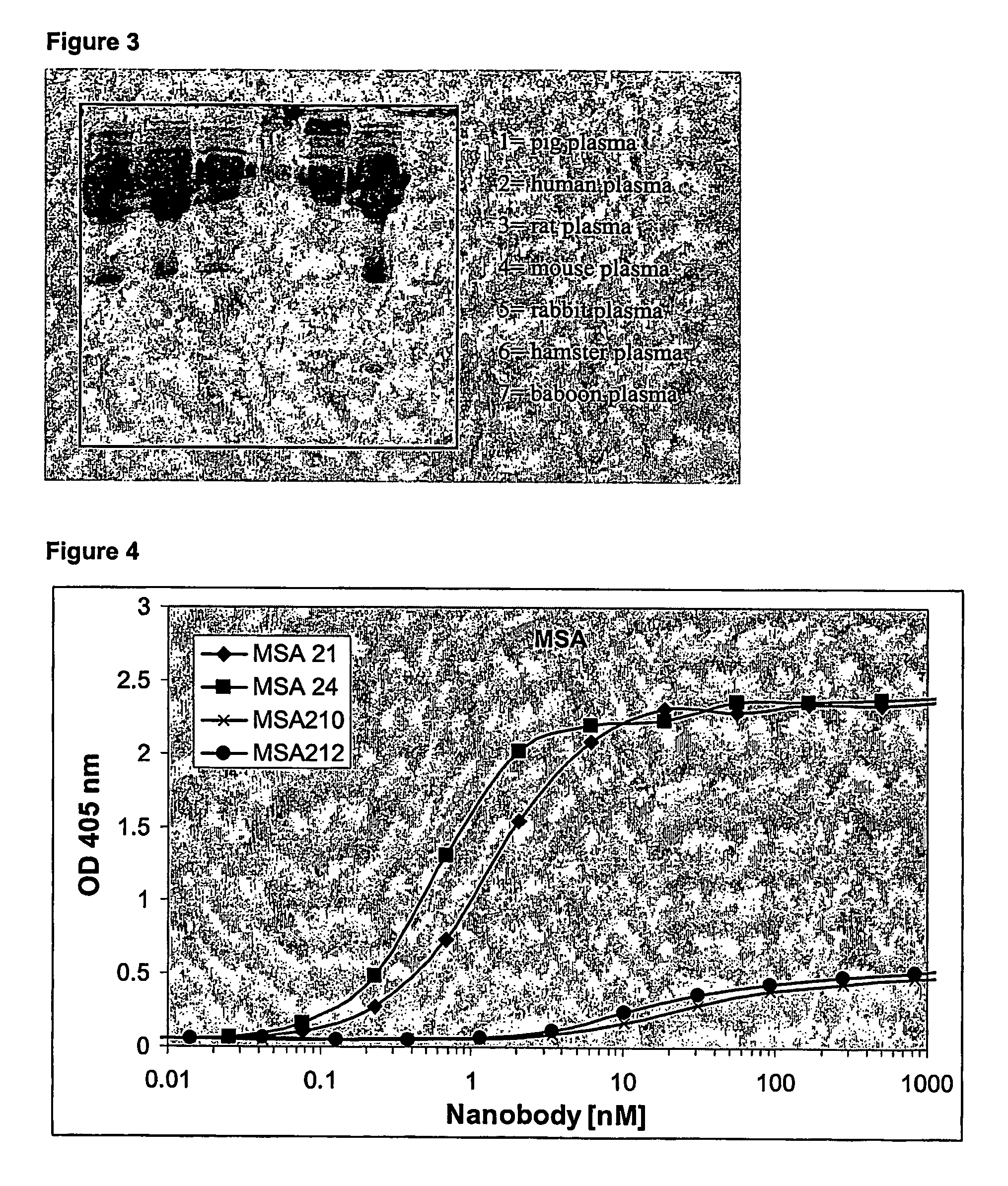
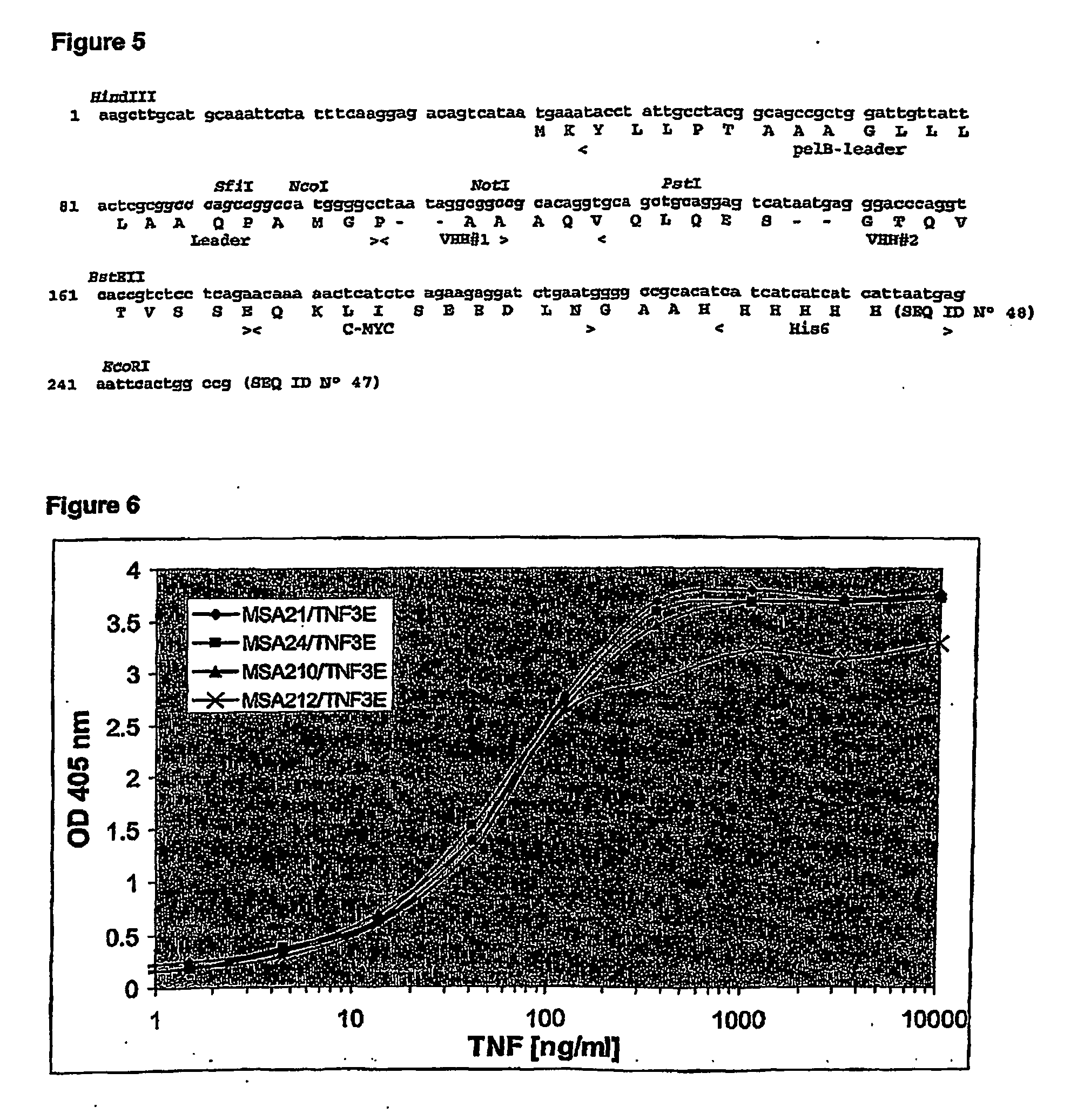
Stabilized single domain antibodies
a single-domain, antibody technology, applied in the direction of antibody medical ingredients, antibody mimetics/scaffolds, fusion polypeptides, etc., can solve the problems of high cost of antibody production in mammalian cells, large protein doses, and discouraged development of traditional antibody therapeutics for these applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunization of Llamas
[0308] One llama was Immunized with human serum albumin (HSA). The immunization scheme is summarized in Table 1.
example 2
[0309] Peripheral blood lymphocytes (PBLs) were isolated by centrifugation on a density gradient (Ficoll-Paque Plus Amersham Biosciences). PBLs were used to exitact total RNA (Chomczynski and Sacchi 1987). cDNA was prepared on 100 μg total RNA with MMLV Reverse Transcriptase (Gibco BRL) using oligo d(T) oligonucleotides. The cDNA was purified with a phenol / chloroform extraction, followed by an ethanol precipitation and subsequently used as template to amplify the VHH repertoire.
[0310] In a first PCR, the repertoire of both conventional (1.6 kb) and heavy-chain (1.3 kb) antibody gene segments were amplified using a leader specific primer (5′-GGCTGAGCTCGGTGGTCCTGGCT-3′) (SEQ ID No 41) and the oligo d(T) primer (5′-AACTGGAAGAATTCGCGGCCGCAGGAATTTTTTTTTTTTTTTTTT-3′) (SEQ ID No 42). The resulting DNA fragments were separated by agarose gel electrophoresis and the 1.3 kb fragment, encoding heavy-chain antibody segments was purified from the agarose gel. A second PCR was...
example 3
Rescue of the Library, Phage Preparation
[0311] The library was grown at 37° C. in 10 ml 2×TY medium containing 2% glucose, and 100 μg / ml ampicillin, until the OD600 nm reached 0.5. M13KO7 phages (1012) were added and the mixture was incubated at 37° C. for 2×30 minutes, first without shaking, then with shaking at 100 rpm. Cells were centrifuged for 10 minutes at 4500 rpm at room temperature. The bacterial pellet was resuspended in 50 ml of 2×TY medium containing 100 μg / ml ampicillin and 25 μg / ml kanamycin, and incubated overnight at 37° C. with vigorously shaking at 250 rpm. The overnight cultures were centrifuged for 15 minutes at 10,000 rpm at 4° C. Phages were PEG precipitated (20% poly-ethylene-glycol and 1.5 M NaCl) and centrifuged for 30 minutes at 10,000 rpm. The pellet was resuspended in 20 ml PBS. Phages were again PEG precipitated and centrifuged for 30 minutes at 20,000 rpm and 4° C. The pellet was dissolved in 5 ml PBS-1% casein. Phages were titrated by infection of TG1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com