Methods of treating cardio pulmonary diseases with NO group compounds
a cardio pulmonary disease and no group compound technology, applied in the field of treating cardio pulmonary diseases with no group compound, can solve the problems of limiting the use of pulmonary disorders, unable to fully avoid nosub>2/sub>2, and producing longer lasting effects, so as to achieve the effect of relieving hypoxemia and selective pulmonary vasodilation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
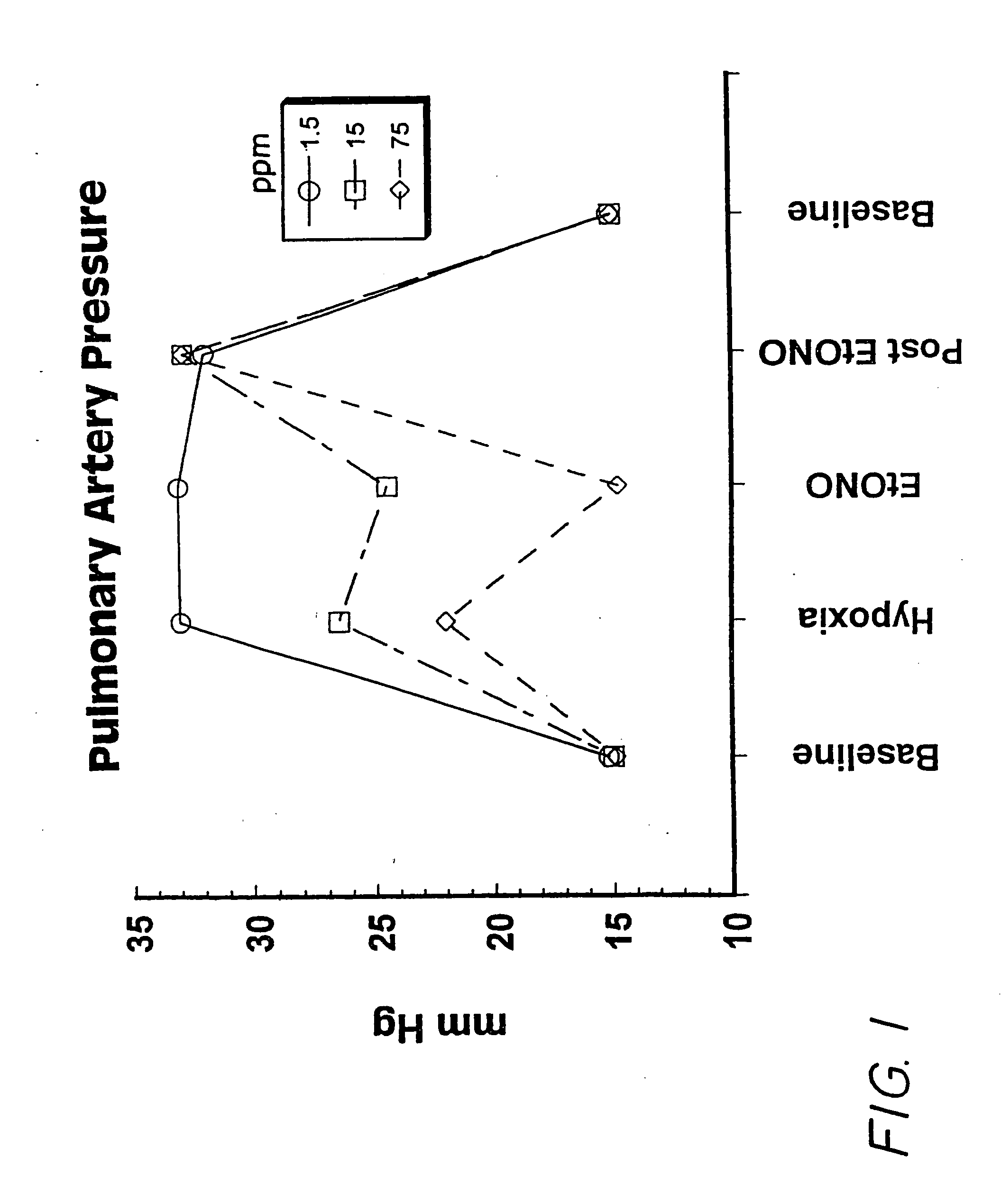
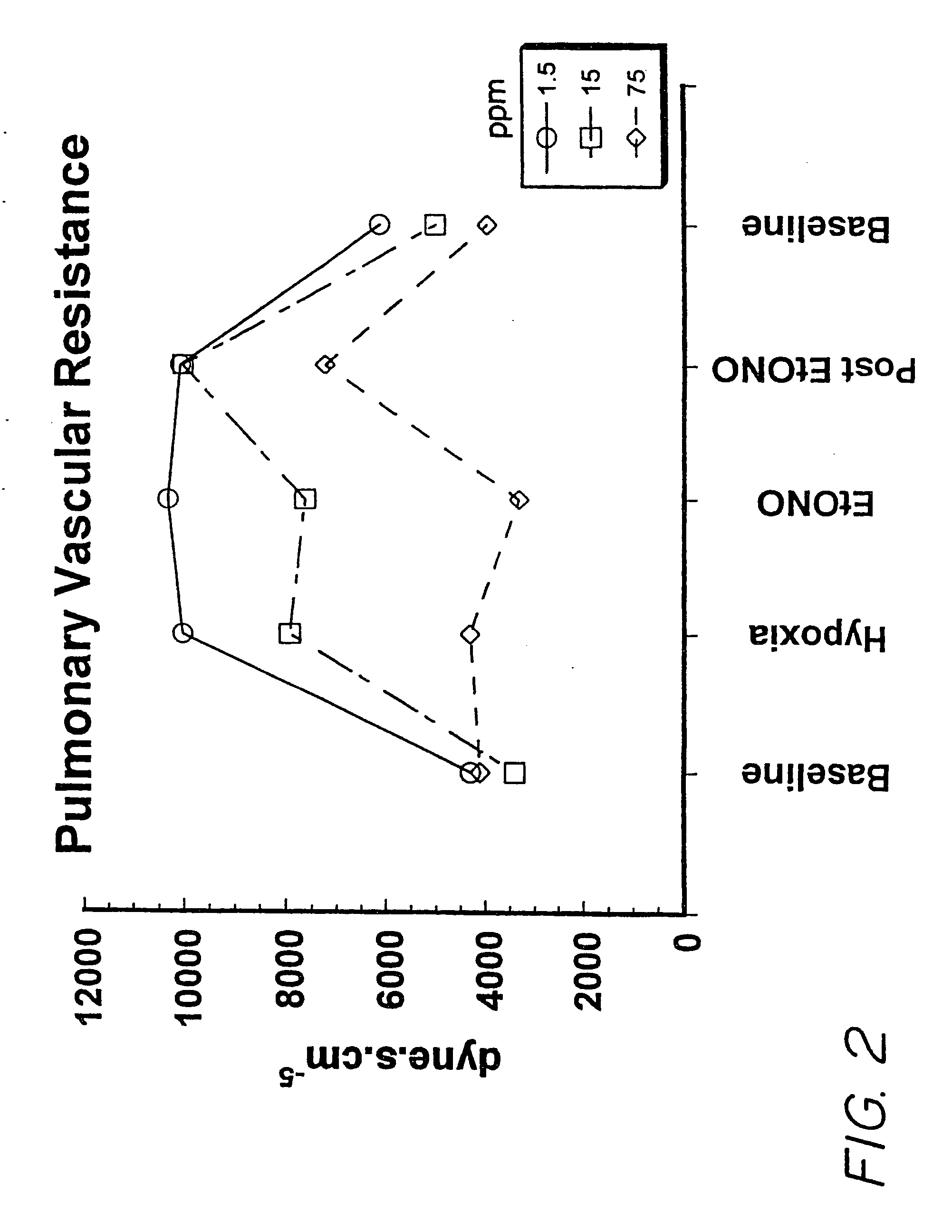
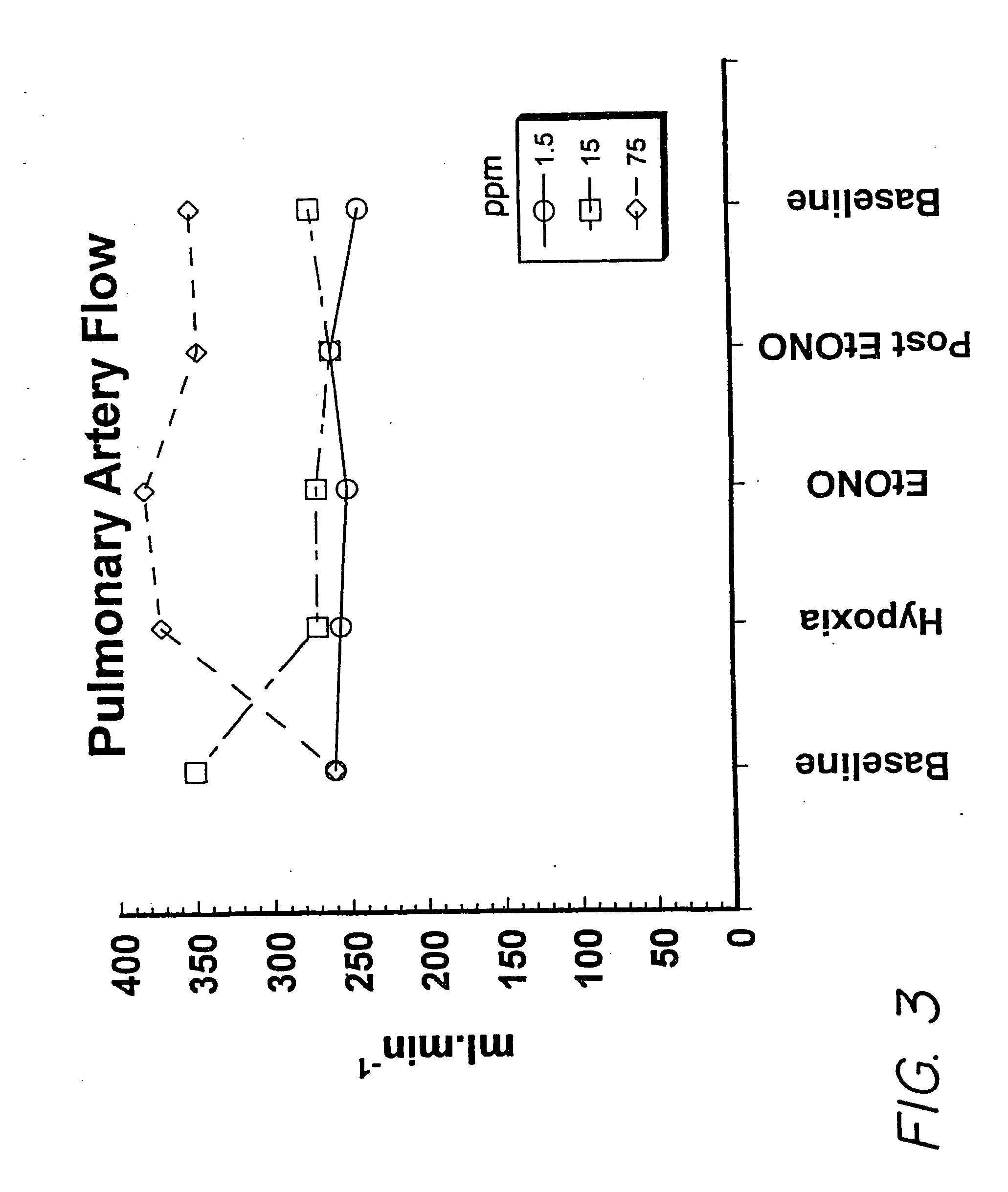
[0090] The experiment was carried out using a pig model of pulmonary hypertension as follows:
[0091] Mixed strain two-three weeks old piglets were utilized. Initial anesthetic induction was by inhaled halomethane 5%, reduced to 2% when the animal was stable. A bolus of 20 μg / kg of fentanyl and 0.2 mg / kg of acepromazine was given after tracheostomy surgery and insertion of a jugular venous line, followed by a continuous fentanyl infusion of 10 μg / kg / hr. An incision in the right side of the neck allowed the insertion of a catheter through the external jugular vein into the right atrium, through which maintenance i.v. fluid of 30 mL / kg / hr of 5% glucose was infused. A catheter was placed in the carotid artery for measurement of systolic arterial pressure (SAP). After the tracheostomy, halothane was discontinued, assisted ventilation was started, and paralysis was obtained using pancuronium bromide (0.1 mg / kg) every 45 minutes. Further bolus doses of fentanyl (5-10 μg / kg) were administer...
example ii
[0107] A 30-year-old white female with pulmonary pressures of 70 / 40 mm Hg is admitted into an intensive care unit and deteriorates due to right heart failure, and is given for inhalation through a face mask an admixture of O2, N2 and ethyl nitrite such that the PaO2 is maintained at 90 and ethyl nitrite is present at 70 ppm. Pulmonary pressures fall to 30 / 15 and right heart failure disappears.
[0108] In another case, an identical patient receives the same treatment except for 80 ppm inhaled NO in place of the 70 ppm ethyl nitrite. Pulmonary pressures drop but the patient develops airway hyperreactivity (slight wheezing) and a chemiluminescence analyzer shows threefold increase in NO2 concentration in exhaled air. Moreover, methemoglobin content in the blood is measured at 10%. The patient is switched from NO to inhaled ethyl nitrite (70 ppm), and NO2 and methemoglobin levels drop and recovery is maintained.
example iii
[0109] A 60-year-old male cancer patient develops radiographic changes consistent with ARDS, post-chemotherapy. The patient's PaO2 falls to 50 mm Hg despite being on 100% oxygen and a right heart catheterization reveals a normal left ventricular endiastolic pressure. The patient is administered 40 ppm inhaled ethyl nitrite. The PaO2 increases to 70 mm Hg.
[0110] An identical patient is given 30 ppm inhaled NO and acute PaO2 improvement occurs but then clinical deterioration occurs characterized by worsening chest X-rays (due to inflammation) and renal impairment and PaO2 drops from 70 to 60 mm Hg. The patent is switched to 50 ppm inhaled ethyl nitrite and the radiographic changes and renal impairment stabilize and PaO2 increases to 90 mm Hg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com