Pharmaceutical Composition And Method For Neoangiogenesis/Revascularization Useful In Treating Ischemic Heart Diseases
a technology of ischemic heart disease and composition, applied in the direction of drug composition, biocide, cardiovascular disorder, etc., can solve the problems of inability to support the greater demands, inability to regenerate myocyte replacement, and rapid growth of fibrous tissue, so as to prevent further ischemic death of cardiomyocytes, promote angiogenesis, and promote the effect of angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
I. Experimental Procedures
[0018]All protocols used in the present invention conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health, and were approved by the Animal Experimental Ethical Committee of The Chinese University of Hong Kong.
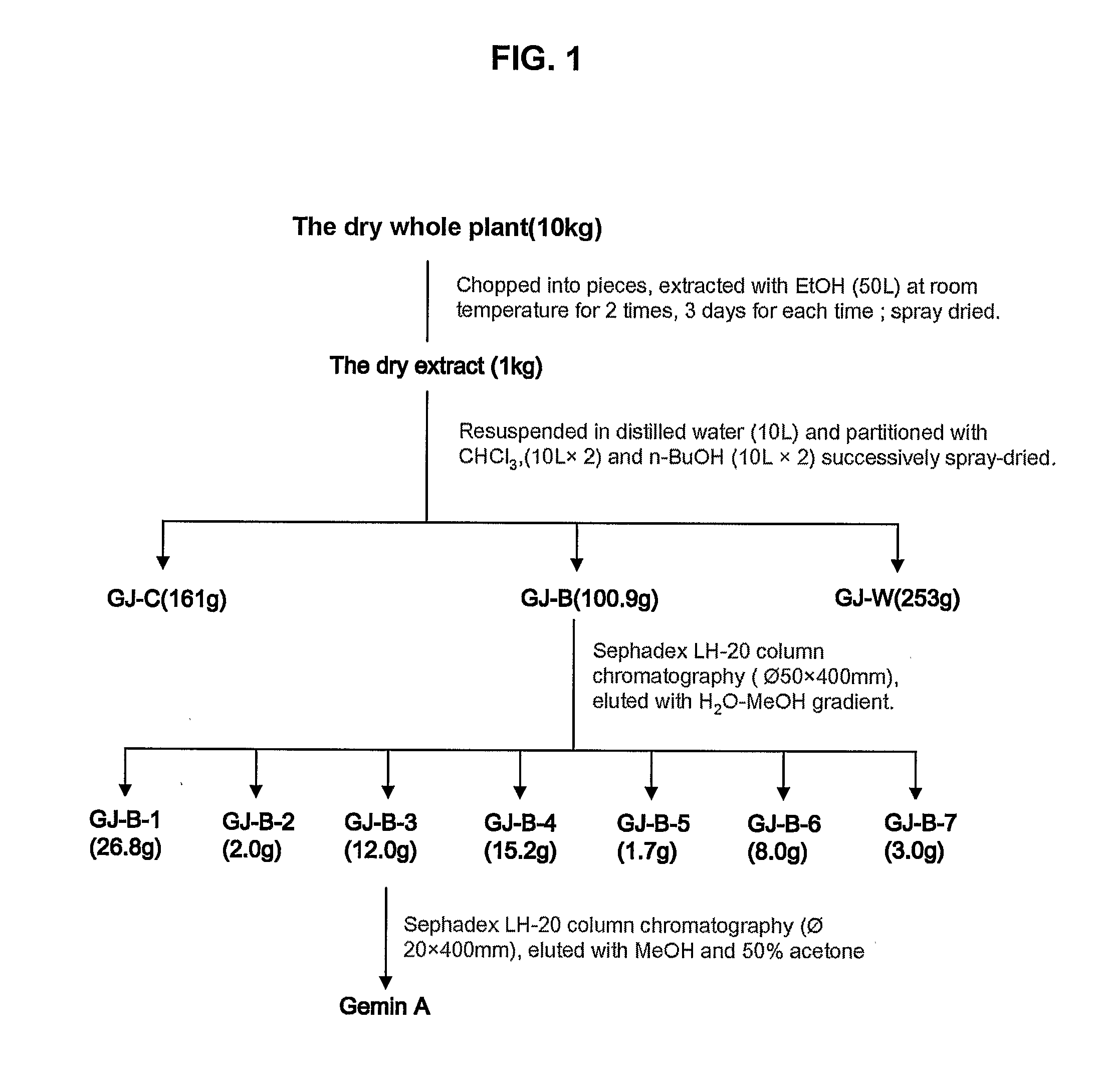
[0019]Isolation of Ga from Geum Japonicum: For the experiments disclosed in the following, Ga was obtained from the plant of Geum Japonicum. Referring to FIG. 1, the plant was collected from Guizhou Province of China in August was dried (10 kg) and percolated with 70% ethanol (100 L) at room temperature for 3 days twice. The extract was combined and spray-dried to yield a solid residue (1 kg). The solid residue was suspended in 10 liter H2O and successively partitioned with chloroform (10 L) twice, then n-butanol (10 L) twice to produce the corresponding fractions. The n-butanol (GJ-B) soluble fraction was filtered and spray dried to yield a powder fraction. It was shown that n-BuOH soluble ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com