Modified starch material of biocompatible hemostasis
a technology of biocompatible hemostasis and modified starch, which is applied in the direction of biocide, drug composition, animal repellent, etc., can solve the problems of high risk of excess blood loss, fever in patients, and human body very slowly absorbs gelatin material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
Preferred Embodiment 1
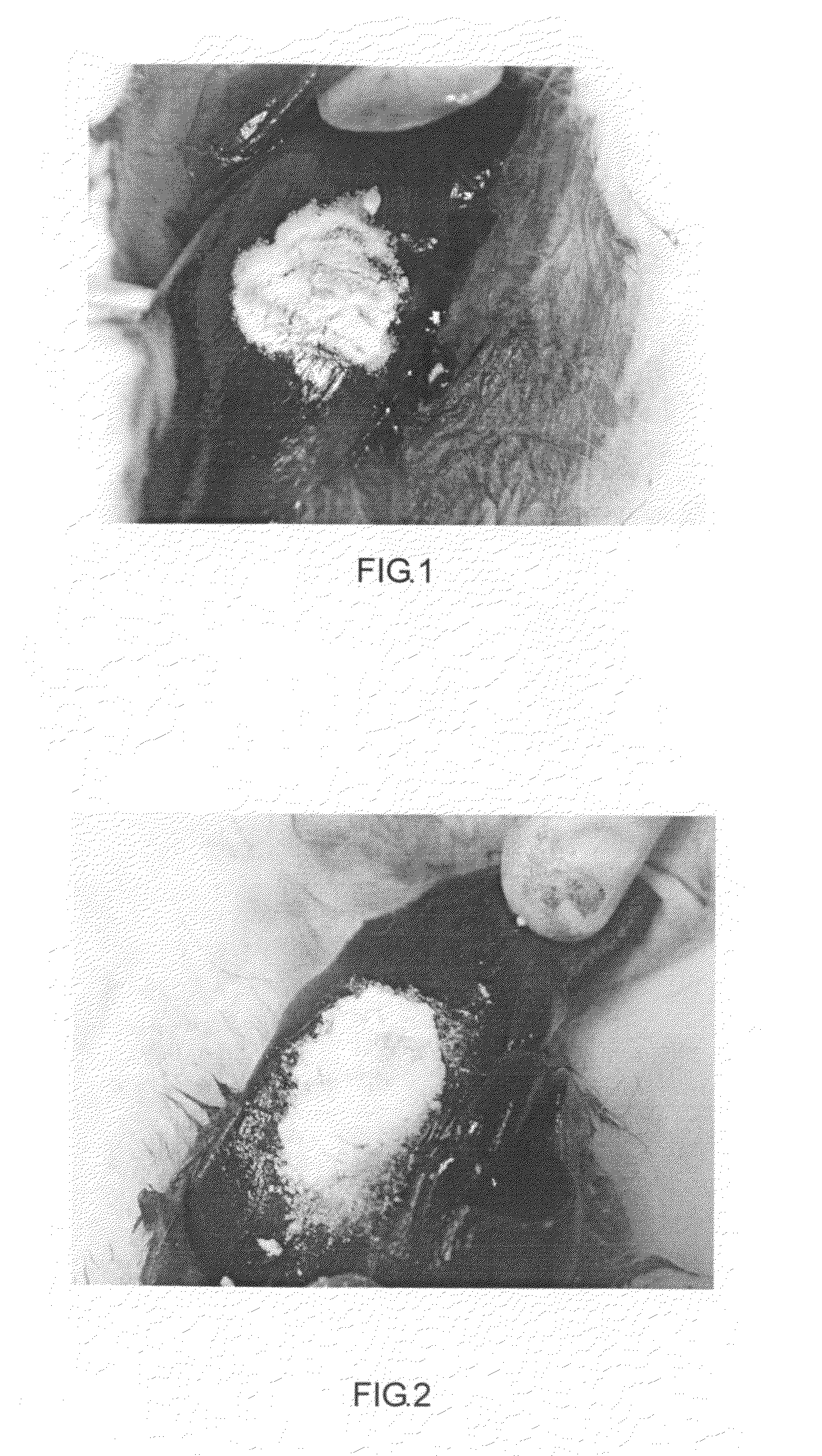
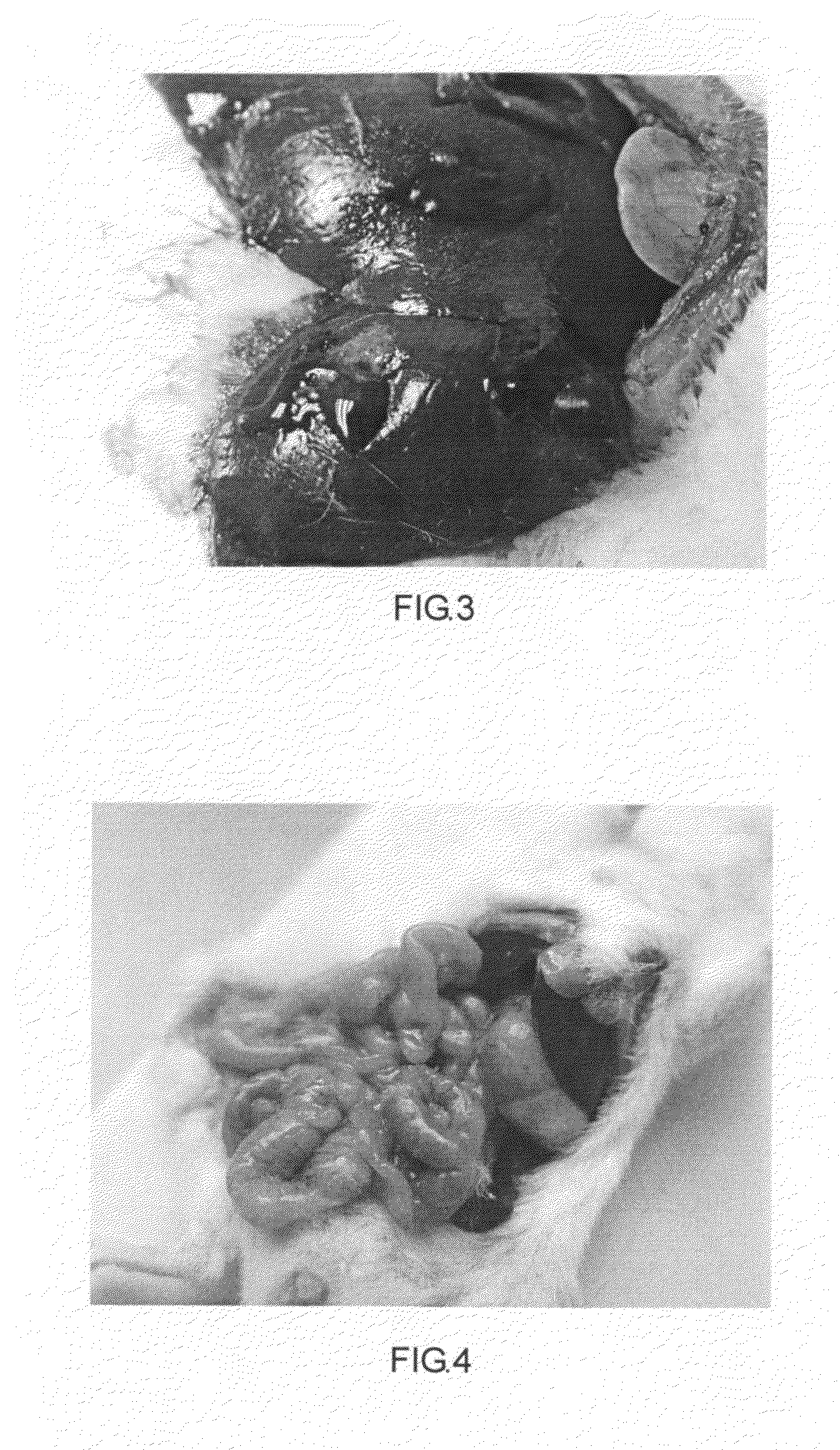
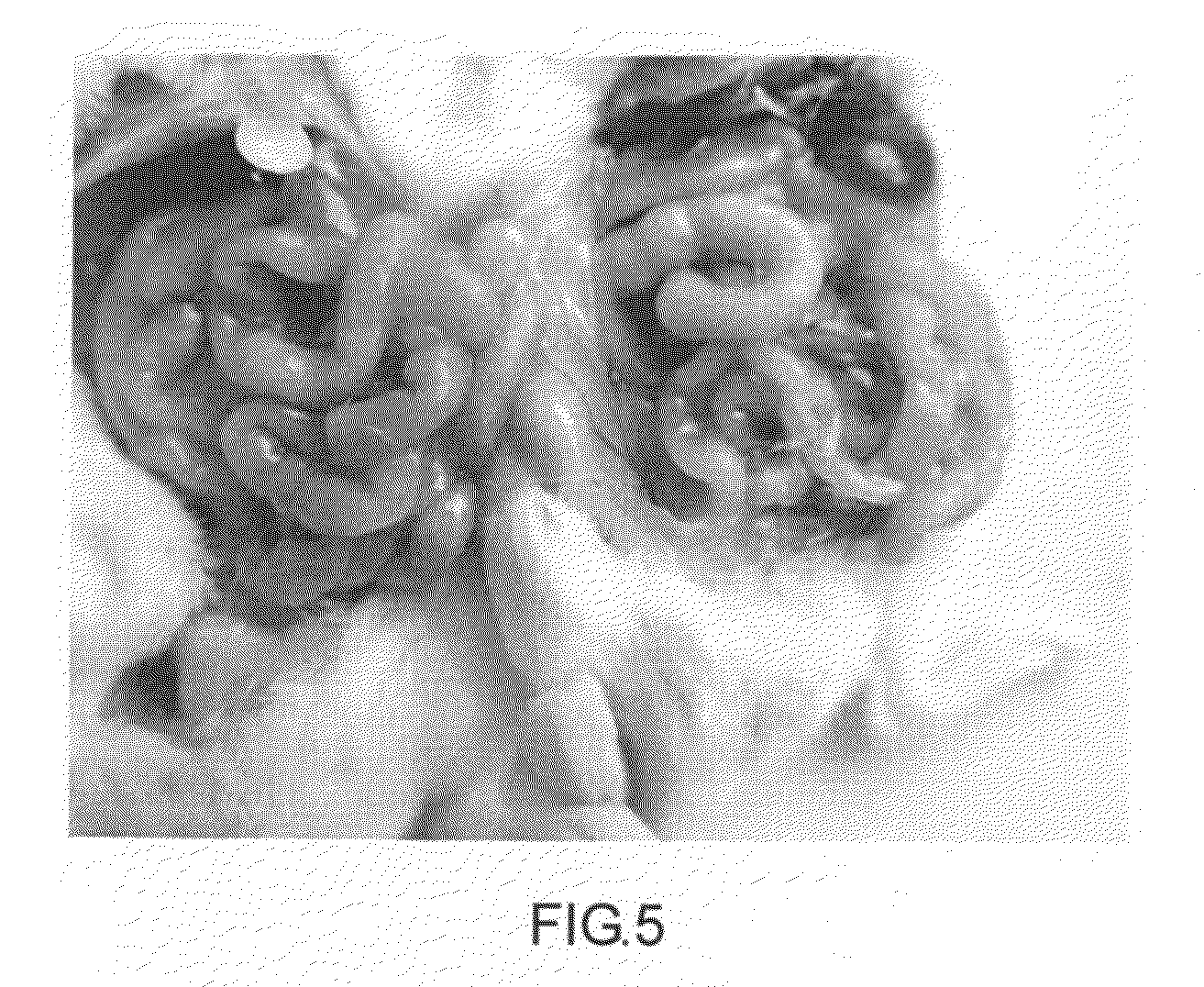
[0185]A biocompatible modified starch material for use as hemostatic material. It includes carboxymethyl starch (66#). Carboxymethyl starch material is added into an agglomerator at 40˜50° C. Distilled water is added. The processes include particles coagulation (agglomeration) and pellet making. Molecule weight of the carboxymethyl starch (66#) is 15,000˜2,000,000 dalton. Diameter of the particles is 10˜1000 μm. Particle diameter is between 30 and 500 μm in no less than 95% of the particles. Viscosity of a 6.67% suspension at 37° C. is 557.9 mPa·s. Work of adhesion under room temperature is 68.1 g·sec when the modified starch is saturated with water.
embodiment 2
Preferred Embodiment 2
[0186]A modified starch absorbable hemostatic material includes hydroxyethyl starch (88#). Hydroxyethyl starch material and distilled water are added into an agglomeratorat 40˜50° C. The processes include particles coagulation (agglomeration) and pellet making Molecule weight of the hydroxyethyl starch (88#) is 15,000˜1,000,000 dalton. Diameter of hydroxyethyl starch (88#) particles is 10˜1000 μm. Particle diameter is between 50 and 500 μm in no less than 95% of the particles. Work of adhesion under room temperature is 420.9 g·sec when the modified starch is saturated with water.
[0187]The water absorption ability of this invention is measured by a capillary method. Water is added into an acid burette so that the liquid level at zero graduation of the acid burette equals to the bottom of the filter plate of a sand core funnel. Filter paper is trimmed into a disc with a 2.25 cm radius, weighed, and put into the sand core funnel until it fully touches the filter p...
embodiment 3
Preferred Embodiment 3
[0204]A biocompatible modified starch material for use as hemostatic material. It includes pre-gelatinized hydroxypropyl distarch phosphate (51#). Its molecule weight is over 15,000 dalton (15,000˜2,000,000 dalton). Diameter of its particles is 10˜1000 μm. Particle diameter is between 50 and 500 μm in no less than 95% of the particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| grain diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com