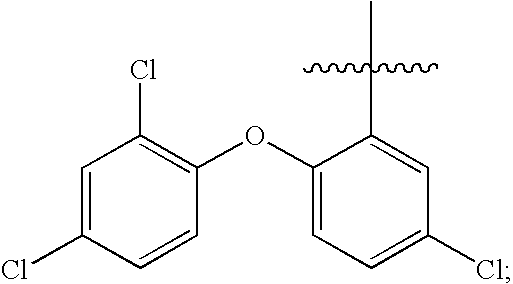
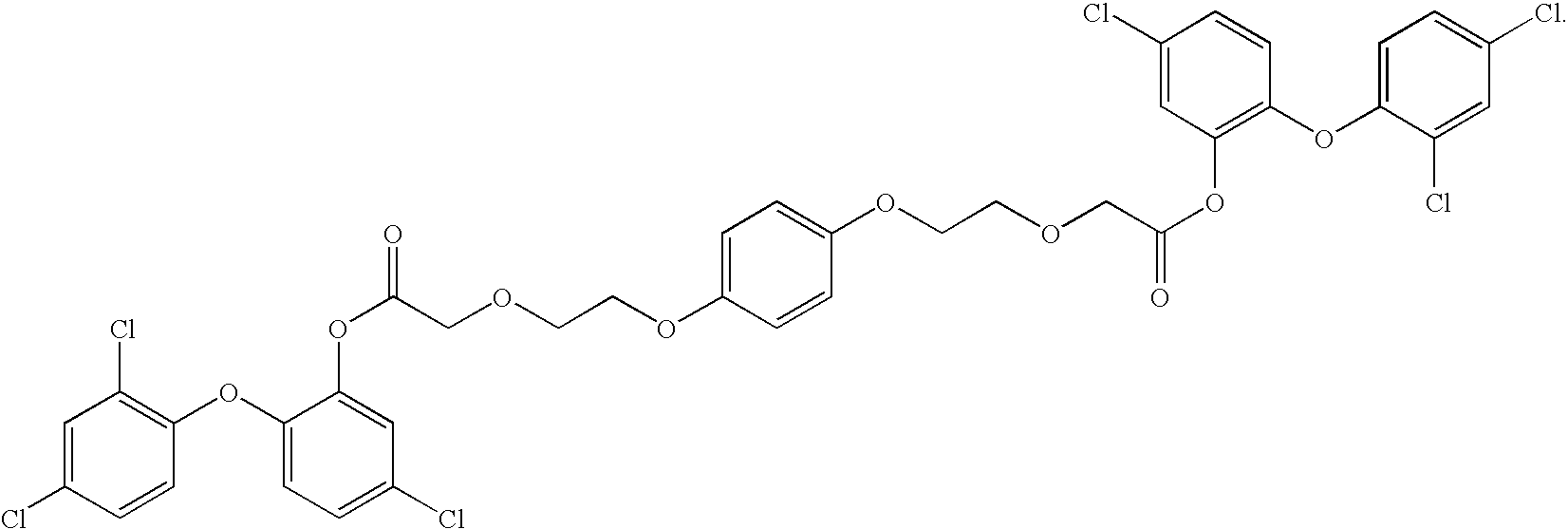
Functionalized biodegradable triclosan monomers and oligomers for controlled release
a technology of triclosan monomers and oligomers, which is applied in the direction of solution delivery, joint implants, infusion needles, etc., can solve the problems of difficult hydrolysis and difficult polymerization in the phenolic state, and achieve the effects of improving bioavailability, high controllability, and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[5-Chloro-2-(2,4-dichloro-phenoxy)-phenoxy]-acetic acid methyl ester
[0175]
[0176]To a mixture of triclosan (50 grams, 173 mmol), anhydrous K2CO3 (100 grams, 723 mmol), sodium iodide (10 grams, 66.7 mmol) and disodium phosphate (10 grams, 70 mmol) in anhydrous acetone (500 mL) was added methyl chloro acetate (30 grams, 276 mmol). The reaction mixture was refluxed for 16 hours. Acetone was distilled and water (600 mL) was added. Crude 1 was extracted into ethyl acetate, dried over Na2SO4, distilled and purified by column chromatography on silica gel using hexane as eluant to give pure 1 (45 grams, 72.1%) as a white powder. m.p: 52-53° C. IHNMR (CDCl3) δ 3.78(s, 3H, ester), 4.65 (s, 2H,OCH2), 6.72(d,1H,Ar), 6.88(m,2H,Ar), 6.92(m,1H,Ar), 7.10(m,1H,Ar), 7.42(s,1H,Ar).
example 2
2-[5-Chloro-2-(2,4-dichloro-phenoxy)-phenoxy]-propionic acid methyl ester
[0177]
[0178]To a mixture of triclosan (50 grams, 173 mmol), anhydrous K2CO3 (100 grams, 723 mmol), sodium iodide (10 grams, 66.7 mmol) and disodium phosphate (10 grams, 70 mmol) in anhydrous acetone (500 mL) was added methyl 2-chloro propionate (32.25 grams, 263 mmol). The reaction mixture was refluxed for 24 hours. Acetone was distilled and water (600 mL) was added. Crude 2 was extracted into ethyl acetate, dried over Na2SO4, distilled and purified by column chromatography on silica gel using hexane as eluant to give pure 2 (35 grams, 54%) as a syrup. IHNMR (CDCl3) δ 1.48(d,3H,CH3), 3.75(s, 3H, ester), 4.72 (q,1H,O—CH), 6.74(d,1H,Ar), 6.90(m,3H,Ar), 7.12(dd,1H,Ar), 7.42(d,1H,Ar).
example 3
6-[5-Chloro-2-(2,4-dichloro-phenoxy)-phenoxy]-hexanoic acid methyl ester
[0179]
[0180]To a mixture of triclosan (50 grams, 173 mmol), anhydrous K2CO3 (100 grams, 723 mmol), sodium iodide (10 grams, 66.7 mmol) and disodium phosphate (10 grams, 70 mmol), in anhydrous acetone (500 mL) was added methyl 6-bromo hexanoate (55 grams, 263 mmol). The reaction mixture was refluxed for 30 hours. Acetone was distilled and water (600 mL) was added. Crude 3 was extracted into ethyl acetate, dried over Na2SO4, distilled and purified by column chromatography on silica gel using hexane as eluant to give pure 3 (60 grams 90%) as a syrup. IHNMR (CDCl3) δ 1.24(m,2H,CH2), 1.58(m,2H,CH2), 1.64(m,2H,CH2), 2.24(t,2H,CH2), 3.65(s,3H, ester), 3.92 (t,2H,OCH2)), 6.60(d,1H,Ar), 6.92(m,2H,Ar), 6.96(d,1H,Ar), 7.05(d,1H,Ar), 7.42(s,1H,Ar).
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| absorbable | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com