Method of removing dissolved iron in aqueous systems
a technology of dissolved iron and aqueous system, which is applied in the direction of quarries waste water treatment, filtration treatment, borehole/well accessories, etc., can solve the problems of long time-consuming and laborious removal of dissolved iron, fluid contamination, fluids may or may not have further practical or economic value, etc., to reduce the detrimental effects of polymers through breakdown and/or viscosity reduction, and accelerate the decomposition of peroxides , the effect o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
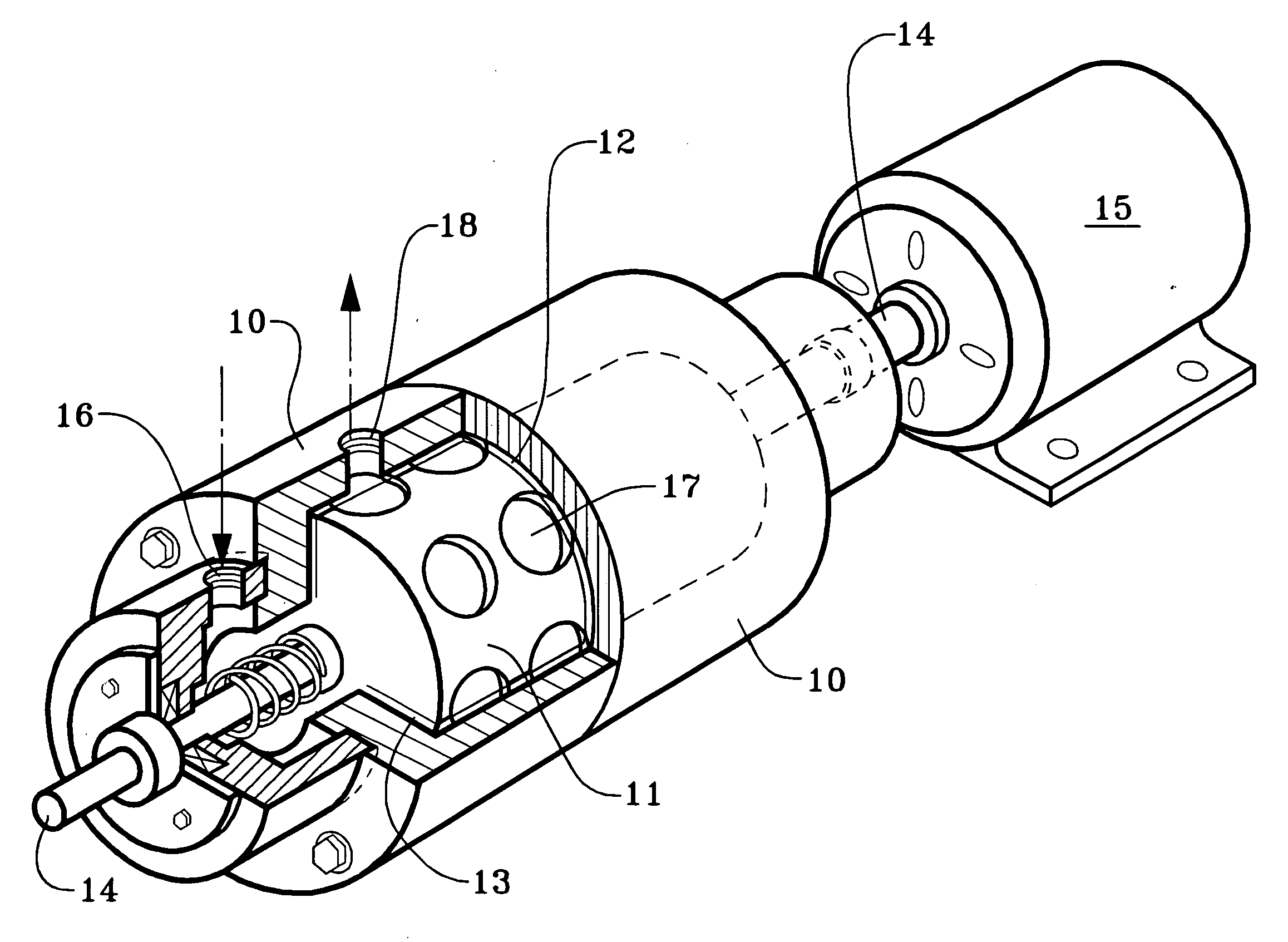
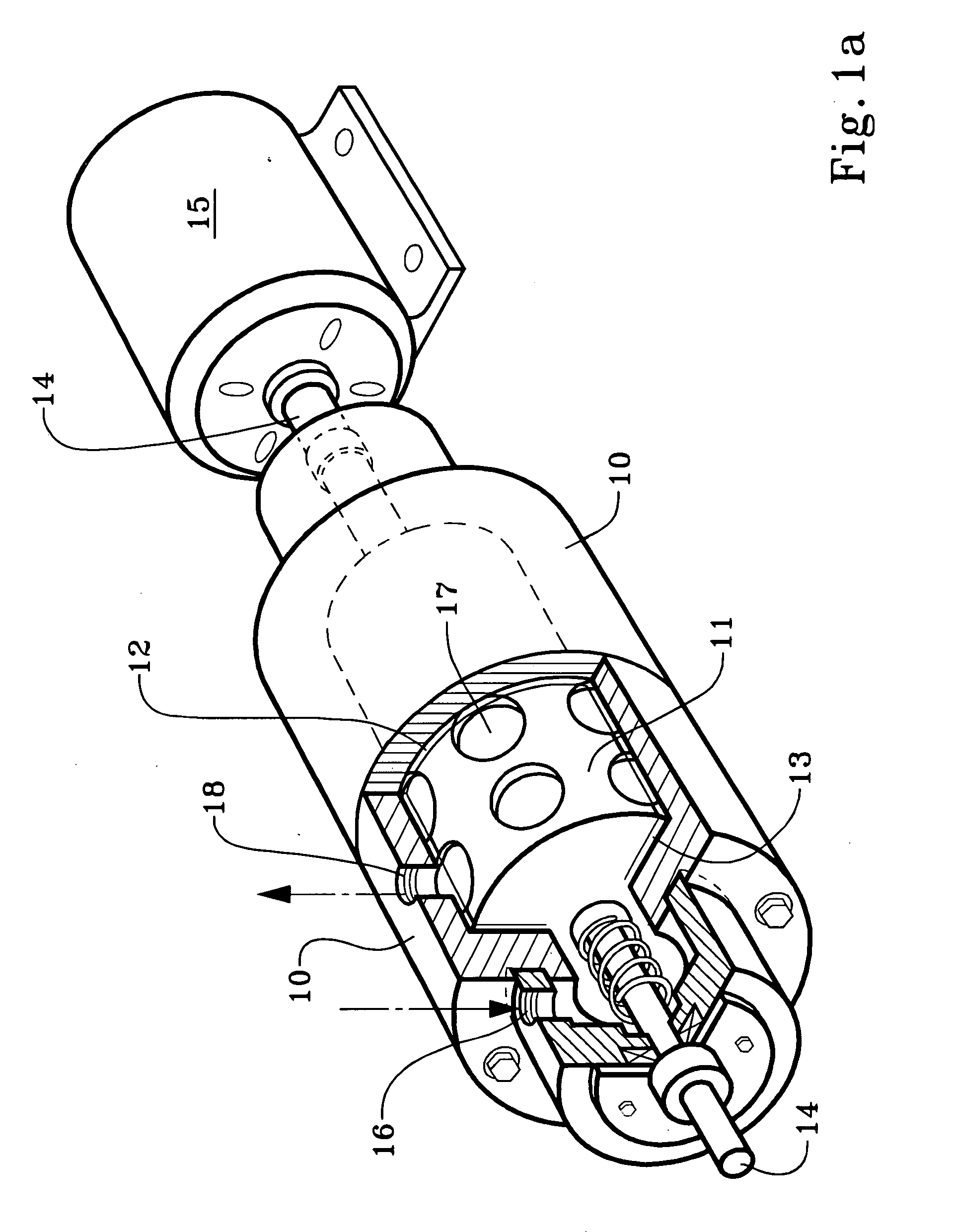
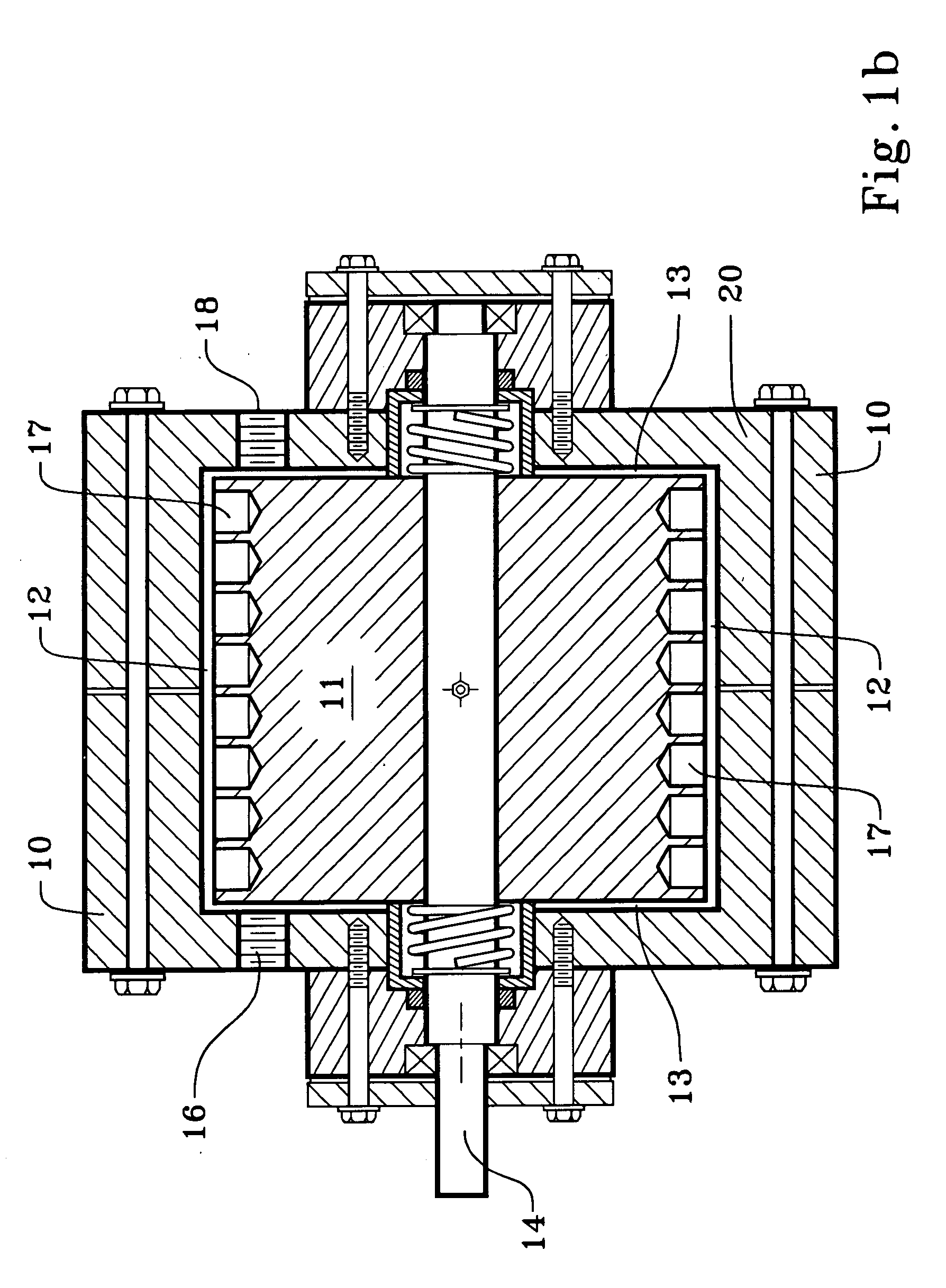
[0021]We use a cavitation device to increase the temperature of the completion, drilling, or workover fluid while also mixing it with an oxidizing agent to oxidize the iron. A cavitation device heats a solution within it by generating shock waves within the solution and also by friction within the device. The term “cavitation” derives from pockets or cavities which are filled by shock waves of fluid.
[0022]We use the term “cavitation device” or to mean and include any device which will impart thermal energy to flowing liquid by causing bubbles or pockets of partial vacuum to form within the liquid it processes, the bubbles or pockets of partial vacuum being quickly imploded and filled by the flowing liquid. The bubbles or pockets of partial vacuum have also been described as areas within the liquid which have reached the vapor pressure of the liquid. The turbulence and / or impact, which may be called a shock wave, caused by the implosion imparts thermal energy to the liquid, which, in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com