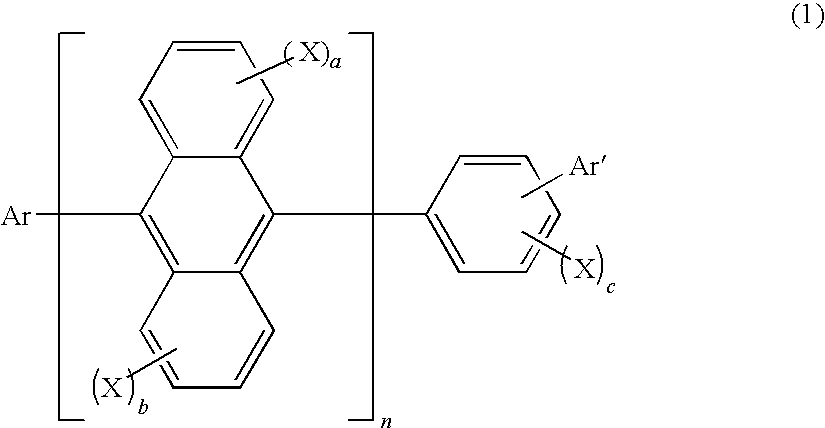
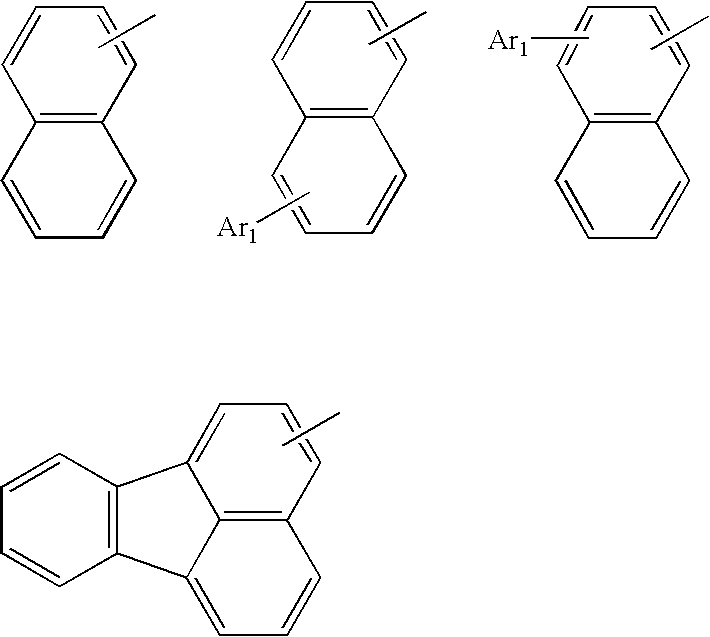
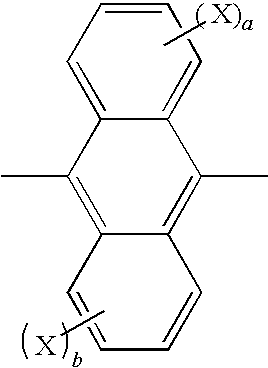
Method for producing aromatic compound and aromatic compound
a technology of aromatic compounds and compounds, applied in the direction of discharge tubes/lamp details, organic chemistry, discharge tubes/lamp details, etc., can solve the problems of insufficient reduction of luminance and initial current efficiency, inability to reduce the contents of bromine compounds and iodine compounds in accordance with conventional processes, and impurities in used materials greatly affect the properties of organic el devices, etc., to achieve the effect of increasing the life of the organic el devi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of 9-(naphthyl-2-yl)-10-(4-(naphthyl-1-yl)phenyl-1-yl)anthracene (BH1)
(1) Synthesis of 9-(naphthyl-2-yl)anthracene
[0127]Under the stream of nitrogen, 30.0 kg of 9-bromoanthracene (manufactured by Tokyo Chemical Industry Co., Ltd.), 24.1 kg of 2-naphthylboronic acid, 2.157 kg of tetrakis(triphenylphosphine)palladium, 48.4 kg of potassium carbonate (manufactured by NIPPON SODA Co., Ltd.), 150 liters of toluene and 150 liters of Solmix were placed into a 1,000 liter reactor, and the reaction was allowed to proceed at 78° C. for 50 hours.
[0128]After the reaction mixture was cooled to the room temperature, 188 liters of tetrahydrofuran and 188 liters of water were added, and the obtained mixture was fractionated. The obtained organic layer was washed with 115 liters of a 5% by mass aqueous solution of sodium hydroxide, 115 liters of a 5% by mass aqueous solution of sodium hydrogencarbonate and 115 liters of a 5% by mass aqueous solution of sodium chloride, successively, and the...
example 1
Treatment of BH1 by the Grignard Reaction
[0140]Under the stream of argon, 600 g of BH1 was suspended in 9 liters of anhydrous tetrahydrofuran (THF). To the resultant suspension, 60 ml of a 32% by mass tetrahydrofuran solution of phenylmagnesium bromide (manufactured by Tokyo Chemical Industry Co., Ltd.) was slowly added dropwise under cooling with ice, and the obtained mixture was stirred at 50 to 60° C. for 30 minutes. To the obtained mixture, a dilute sulfuric acid was added to decompose the unreacted substances. The reaction product was treated by extraction with toluene, and the organic layer was washed with a 5% by mass aqueous solution of NaOH, a 5% by mass aqueous solution of sodium hydrogencarbonate and a 10% by mass aqueous solution of sodium chloride and concentrated under a reduced pressure. Toluene was added to the residue of the concentration to form a material fluid. The material fluid was purified using a column packed with silica gel and alumina, and the obtained flu...
example 2
Treatment of BH1 by the Reaction with an Organolithium Reagent
[0143]Under purging with nitrogen, 600 g of BH1 was dissolved into 5 liters of anhydrous toluene and 5 liters of anhydrous ether. To the obtained solution, 75 ml of a 15% by mass hexane solution of n-butyllithium (manufactured by Tokyo Chemical Industry Co., Ltd.) was slowly added at −78° C. The reaction mixture was stirred at 0° C. for 1 hour, and then the reaction was stopped by adding water. The reaction product was treated by extraction with toluene. The organic layer was washed with a 5% by mass aqueous solution of NaOH, a 5% by mass aqueous solution of sodium hydrogencarbonate and a 10% by mass aqueous solution of sodium chloride and then concentrated under a reduced pressure. Toluene was added to the residue of the concentration to form a material fluid. The material fluid was purified using a column packed with silica gel and alumina, and the obtained fluid was concentrated under a reduced pressure. When a slurry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com