Stable compositions for nucleic acid amplification and sequencing
a technology of nucleic acid amplification and composition, applied in the field of molecular and cellular biology, can solve the problems of inability to amplification and sequence the nucleic acid strand in time, the error rate is somewhat higher, and the dual activity of certain dna polymerases is a drawback for some in vitro applications, so as to maintain enzyme activity for extended periods of tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation of Standard Compositions
[0075]As an initial step in formulating stable, ready-to-use reagent compositions for nucleic acid amplification and sequencing, components were mixed in varying amounts as shown in Table 1 to provide 24 different formulations. The pH on all formulations was adjusted to about 8.3. After filtration through a low protein-binding filter, all compositions were formulated with 20 units / ml of Taq polymerase and were suitable for use as standard compositions for amplification or sequencing of nucleic acid fragments up to about 5-6 kilobases in size.
TABLE 1FORMULATIONS OF STANDARD COMPOSITIONSFormulation Number12345678Tris-HCl,20 mM20 mM20 mM20 mM20 mM20 mM20 mM20 mMpH 8.4KCl50 mM50 mM50 mM50 mM50 mM50 mM50 mM50 mM(NH4)2SO4 0 0 0 0 0 0 0 0dNTP200 μM200 μM200 μM200 μM200 μM Na200 μM Na200 μM Li200 μM LiMgCl21.5 mM1.5 mM1.5 mM1.5 mM1.5 mM1.5 mM1.5 mM1.5 mMDetergents0.004%0.1%0.1%0.1%0.1%0.004%0.1%0.004%Tween20 / NP40Tween20 / NP40Tween20 / NP40Tween20 / NP40Tween20...
example 2
Stability of Standard Compositions
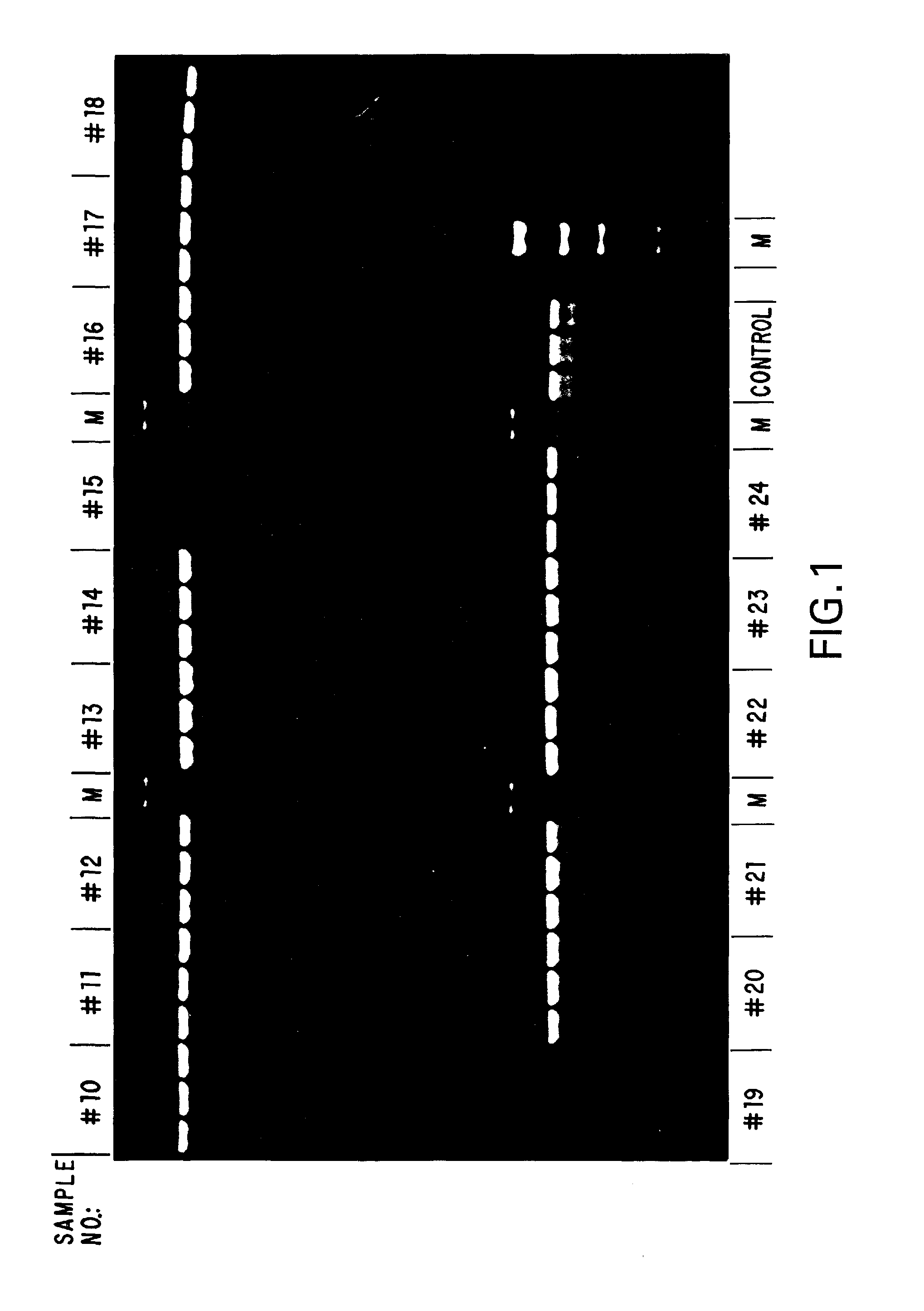
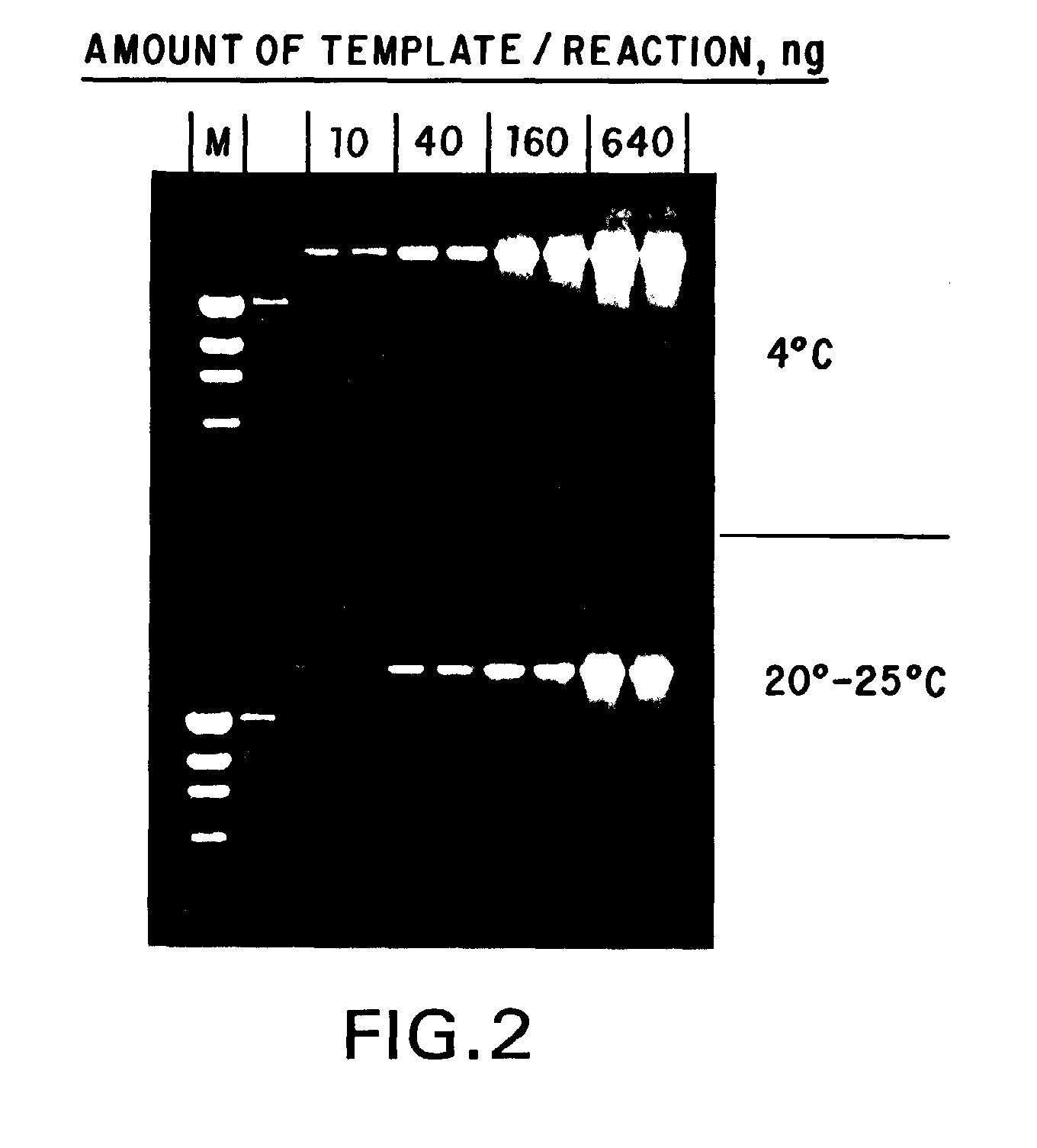
[0076]To examine the stability of the standard compositions formulated in Example 1, samples of each formulation were aliquoted and stored, under diminished light, at ambient temperature (about 20°-25° C.), 4° C., −20° C. and −70° C. Samples of each formulation from each temperature were taken daily for the first week, and weekly thereafter, and used in stability assays. These stability assays were performed by amplifying, via standard PCR, suboptimal amounts of human genomic DNA as a template using a template titration (if few samples were to be compared in a time point) or a fixed amount of template (if a larger number of samples were to be compared). To the desired amount of template DNA in a given formulation, 10 picomoles of primer was added, and the reaction mixtures were subjected to 35 cycles of PCR of 30 seconds at 94° C., 30 seconds at 55° C. and 1 minute / kilobase at 72° C. A portion of each reaction was then subjected to agarose gel elect...
example 3
Formulation and Stability of Large Sequence Compositions
[0083]For use in amplification and sequencing of nucleic acid fragments larger than 5-6 kilobases, it has been suggested as described above that a mixture of Taq and VENT™ or DEEPVENT™ polymerases (U.S. Pat. No. 5,436,149; Barnes, Id.), or of Tth and either Tli, Pyrococcus or Tma (U.S. Pat. No. 5,512,462), be used. Accordingly, Taq and DEEPVENT™ DNA polymerases were formulated, at activity ratios of 100:1 (for samples 1-3) or 50:1 (for sample 4) into solutions containing the buffer salts, cofactors and detergents shown in Table 5. Each of these formulations was adjusted to about pH 9.1, which is optimal for the activity of DEEPVENT™ DNA polymerase (Bej and Mahbubani, Id.). Samples were then stored at about 20-25° C. or at 4° C. and assayed weekly for 12 weeks, and monthly thereafter, in stability assays in which a 20 kilobase target in 100 nanograms of human genomic template was amplified by PCR. Reaction mixtures included 10 p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com