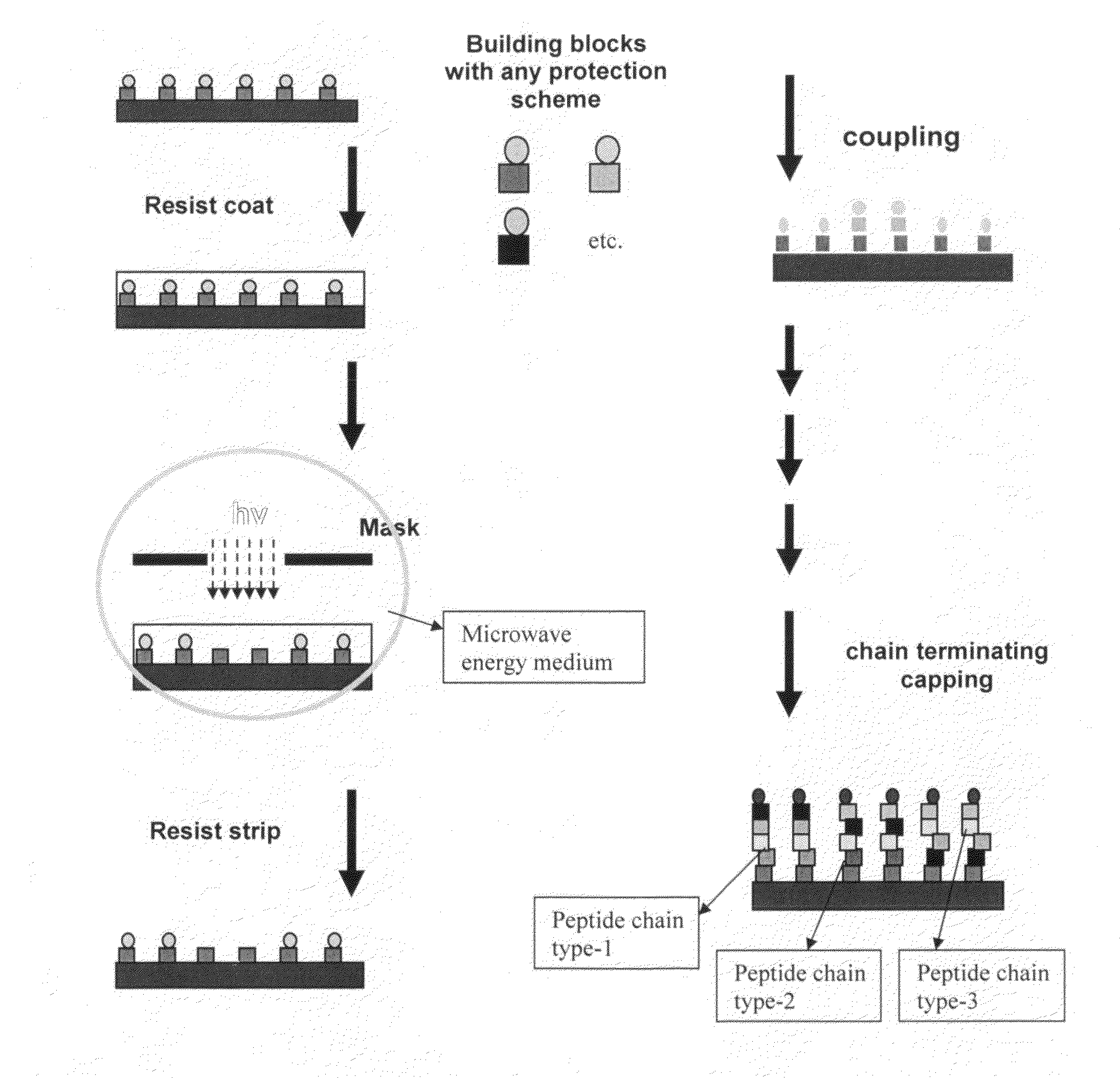
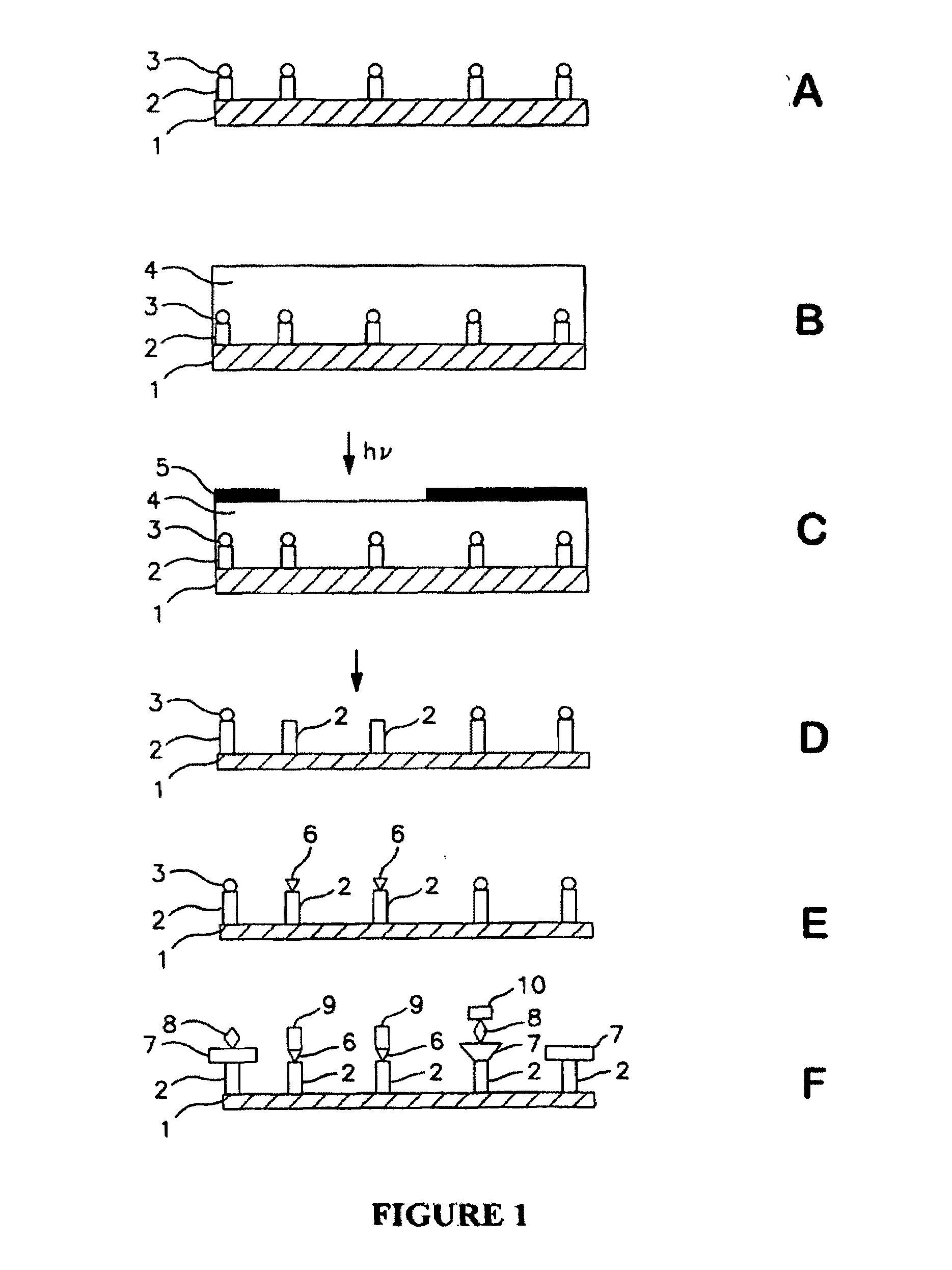
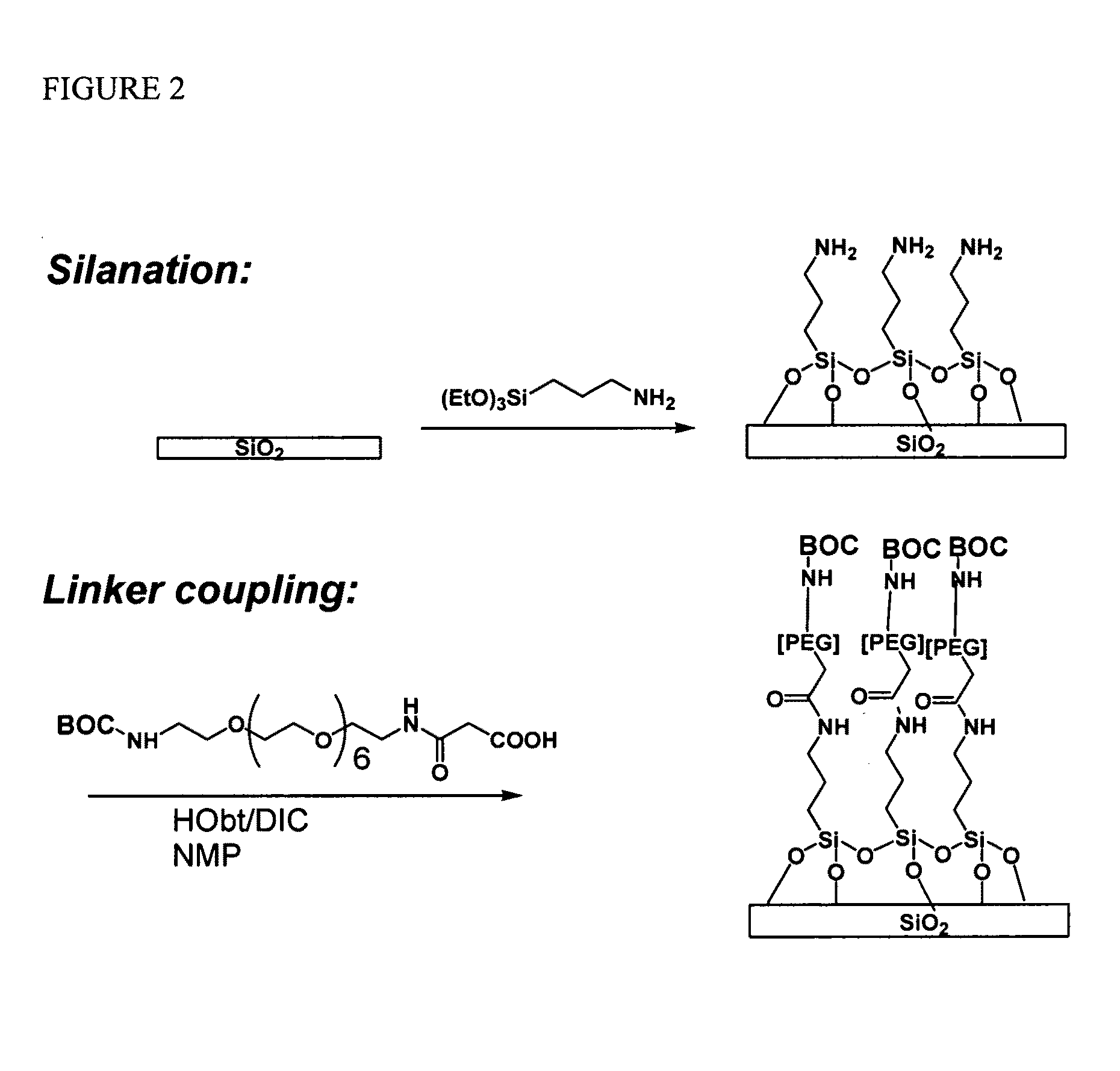
Molecular microarrays and helical peptides
a helical peptide and microarray technology, applied in the field of semiconductor lithography, can solve the problem of random conformation of peptides synthesized in vitro and on solid surfaces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0054]A glass substrate was silanated using a solution of 5% APTES (aminopropyl triethoxy silane) in 95% ethanol. The surface of the substrate was then washed and annealed at about 100° C. for about 1 hour. The substrate was then treated with a 5% solution of DIEA (diisopropyl ethyl amine) in DMF (dimethylformamide). A spacer molecule was then coupled by submerging the surface into a solution of O—(N-Boc-2-aminoethyl)—O′—(N-diglycolyl-2-aminoethyl) hexaethyleneglycol, 0.1 M HOBt, and 0.1 M DIC (diisopropylcarbodiimide) in NMP (N-methylpyrrolidone) with gentle agitation for about 30 min. After coupling was complete, the surface was washed with NMP. Unreacted amine groups on the surface were capped by treatment with 2% acetic anhydride in DMF solution for about 30 minutes. The surface was then washed with DMF and isopropanol.
[0055]A photoresist was prepared by mixing about 2.5% by mass of PMMA, 10% by mass of Bis(4-tert-butylphenyl)iodonium triflate and 10% by mass of isopropyl-9H-thi...
example 2
[0058]An array of wildtype (SDLHKL) and mutant (AGLHKL) peptide was synthesized on an aminated glass surface with a linker molecule, O—(N-Boc-2-aminoethyl)—O′—(N-diglycolyl-2-aminoethyl) hexaethyleneglycol, for spacing the peptides from the surface. The peptides were synthesized in a checkerboard pattern using uniform photodeprotection of t-Boc protecting groups through an open grid mask till the second leucine and spatially localized deprotection through a checkerboard mask for the last two amino acid couplings.
[0059]The photodeprotection and coupling of linker molecules and amino acids was carried out as described in Example 1.
[0060]The peptide array was incubated for 1 hour with 5 μg / ml monoclonal antibody known to specifically recognize the SDLHKL epitope of human p53 protein. A second incubation was performed with fluorescein-labeled rabbit antibody raised against mouse antibody at a 1:100 dilution in phosphate buffered saline with 0.05% Tween 20. A fluorescent checker board pa...
example 3
[0061]Photoresist formulations may include a sensitizer in addition to the photogenerated acid catalyst to generate the acid deprotection catalysts. In general, the amount of PMMA in the resist in these exemplary formulations may vary between about 3% and about 50%.
[0062]Useful photoresists may be made using diaryliodonium salts (DAI) and photosensitizers. The mass ration between DAI and photosensitizer may be between about 1:10 and 1:1. For instance, (toluylcumyl)polonium tetrakis (pentafluorophenyl) borate with isopropyl-9H-thioxanthen-9-one may be formulated in a 1:10 or 1:1 (or a ratio there between) in PMMA and PGMEA to form final concentrations of between about 0.5% to 10% by mass DAI. The formulation selected may be spun coated on the substrate surface and baked. The radiation exposure dose may be between about 0.02 J and about 10 J. Post exposure baking may be conducted for about 30 to 60 seconds at about 40° C. to about 85° C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com