Method and system for the treatment of chronic obstructive pulmonary disease with nebulized anticholinergic administrations
a nebulized anti-cholinergic and chronic obstructive pulmonary disease technology, applied in the direction of heterocyclic compound active ingredients, aerosol delivery, dispersed delivery, etc., can solve the problems of blood oxygen drop, blood waste gas rise, and ultimate collapse of airway walls, so as to reduce or accept side effects, high efficiency, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Single-Dose, Dose Escalation Study to Assess Glycopyrrolate Inhalation Solution (GIS) Using a High Efficiency Nebulizer in Patients with COPD
[0193]Objectives:
[0194]The objectives of the study were as follows: Primary: To assess the safety and tolerability of single ascending doses of glycopyrrolate inhalation solution when administered using a high efficiency nebulizer in patients with COPD. Secondary: (1) To assess and to compare the magnitude and duration of bronchodilator response in patients with COPD following single doses of glycopyrrolate inhalation solution when administered using a high efficiency nebulizer and a conventional jet nebulizer; and (2) To assess the pharmacokinetic (PK) profile of an inhaled glycopyrrolate solution formulation.
[0195]Methodology:
[0196]This two part study was a two-center, single dose, dose-escalation study in patients with COPD of 40-75 years of age. There were 12 subjects included in the study.
[0197]Part 1 was an open label study to assess the ...
example 2
Randomized, Double-Blind, Placebo-Controlled Cross-Over, Single Dose Study
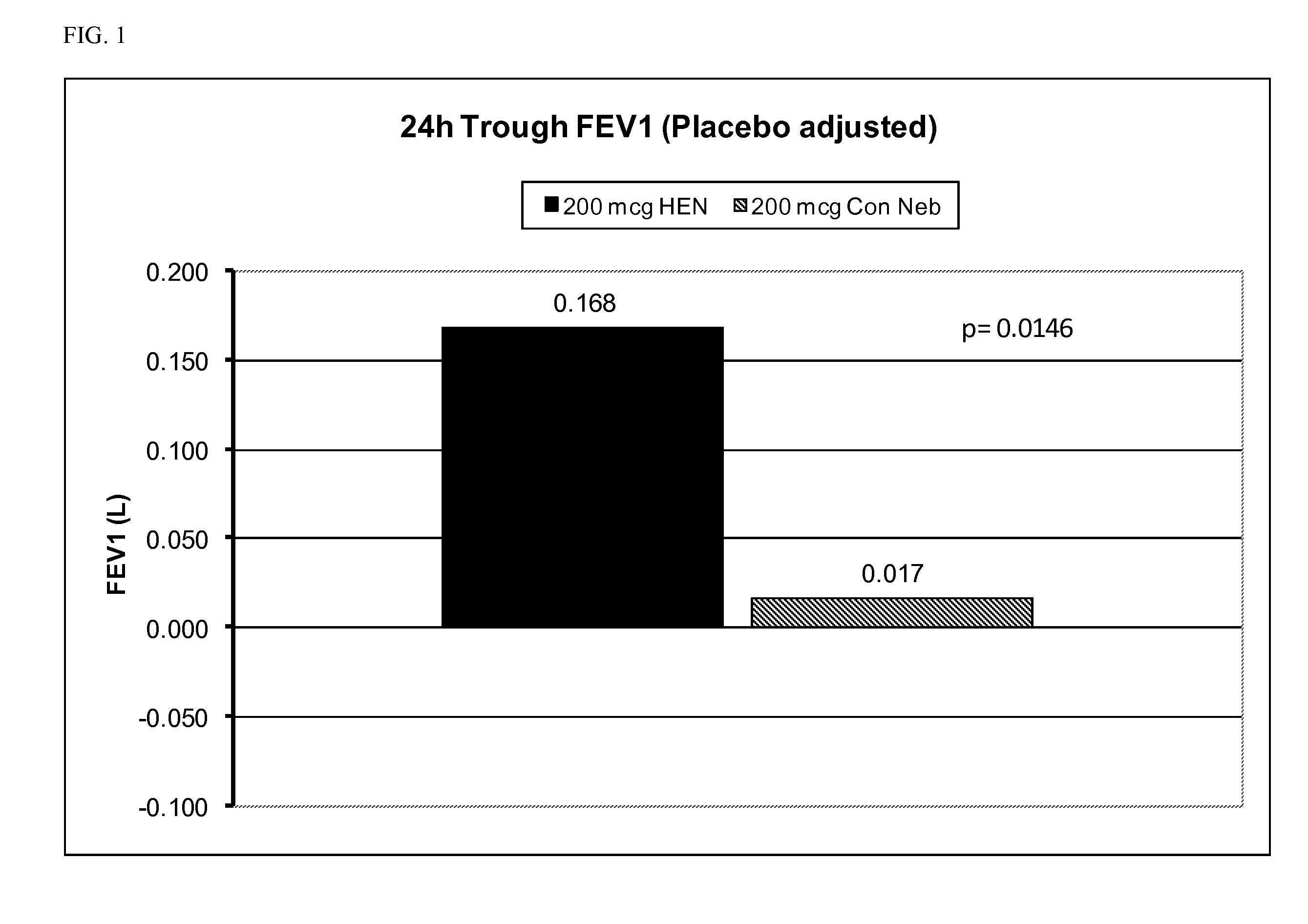
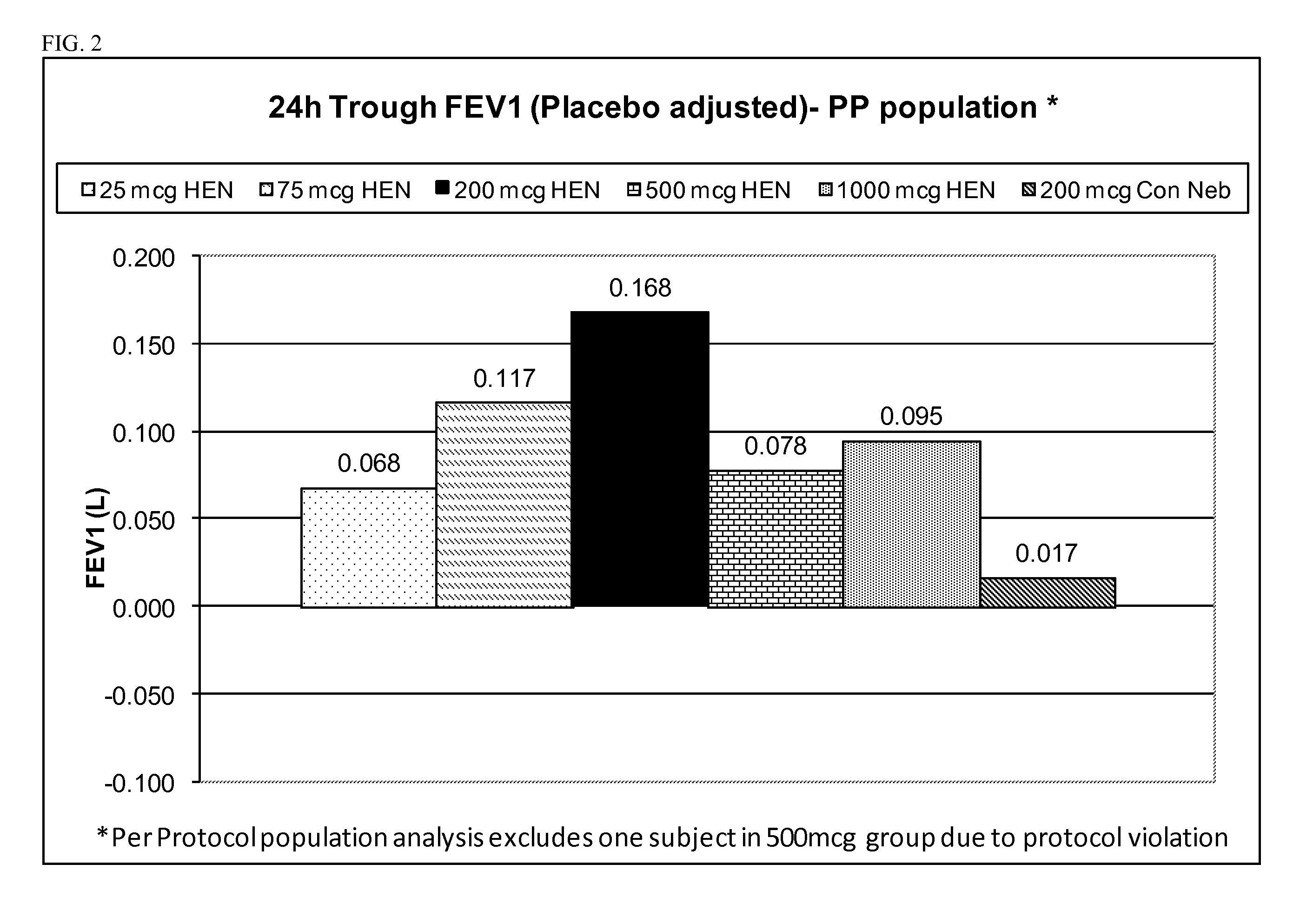
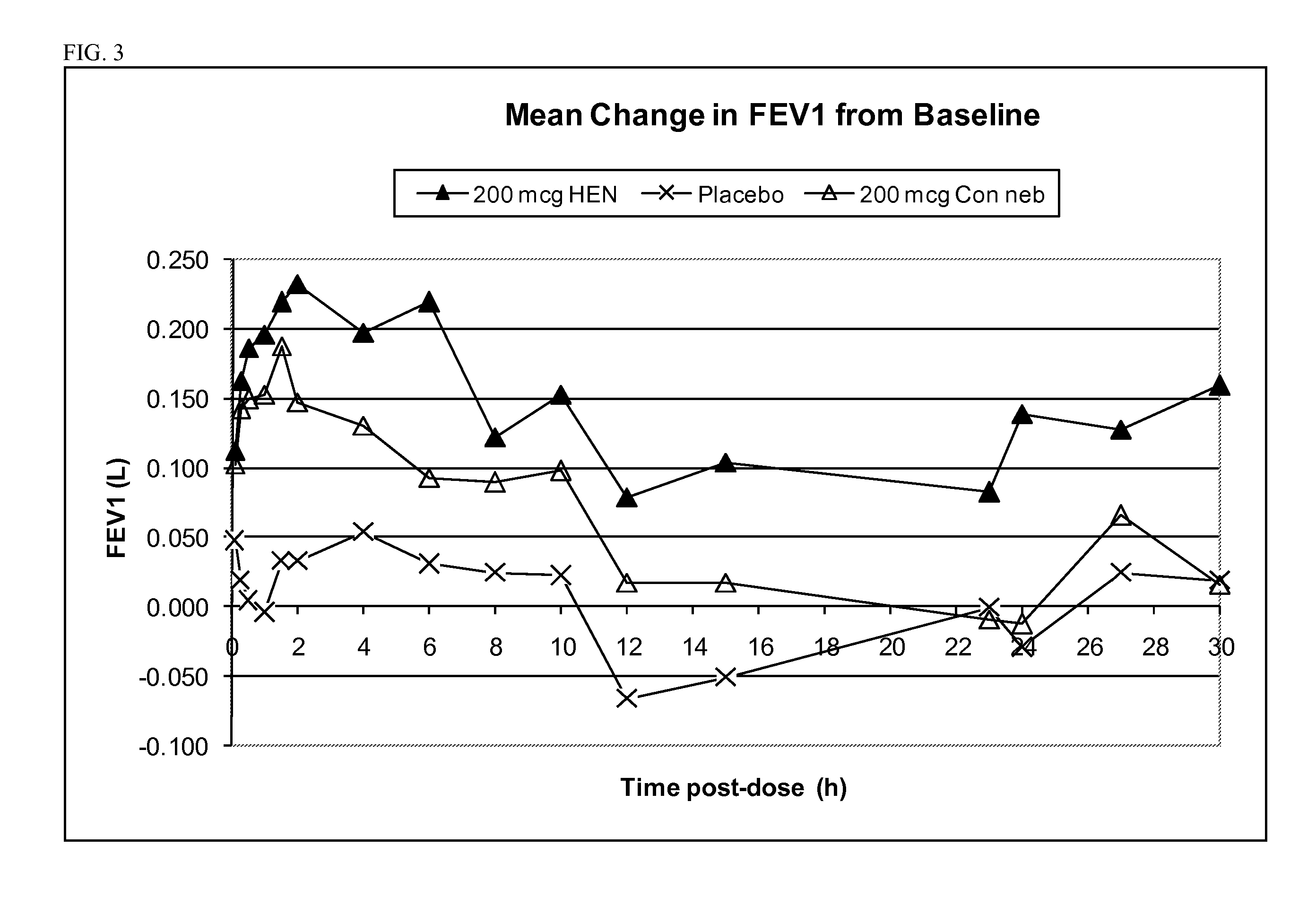
[0220]At least about thirty (30) adult human COPD patients of ages >45 years are randomized to one of six treatment groups: (1) 25 μg glycopyrrolate administered with a high efficiency nebulizer; (2) 50 μg of glycopyrrolate administered with a high efficiency nebulizer; (3) 100 μg of glycopyrrolate administered with a high efficiency nebulizer; (4) 200 μg of glycopyrrolate administered with a high efficiency nebulizer; (5) 500 μg of glycopyrrolate administered with a high efficiency nebulizer; (6) placebo administered with a high efficiency nebulizer.
[0221]Lung function is determined by spirometry, which measures e.g. FEV1 and other suitable spirometry parameters. Spirometry is conducted immediately before and at predetermined intervals following administration of the glycopyrrolate to the patients. Additionally, the patients will be monitored for any adverse events, as well as for vital signs and electrocardi...
example 3
Aerosol Characterization of Glycopyrrolate Inhalation Solution in a High Efficiency Nebulizer and a Conventional Jet Nebulizer
[0224]Objective
[0225]The object of the study was to determine and compare the drug delivery efficiency of two different nebulizer systems, a high efficiency nebulizer and a conventional jet nebulizer, using a glycopyrrolate inhalation solution (GIS). Droplet size and respirable fraction of the aerosol were measured by laser diffraction, while delivered dose, nebulization time and nebulizer residue were assessed by breath simulation using a standard adult breathing pattern.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com