Fluoroalkyl ether sulfonate surfactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
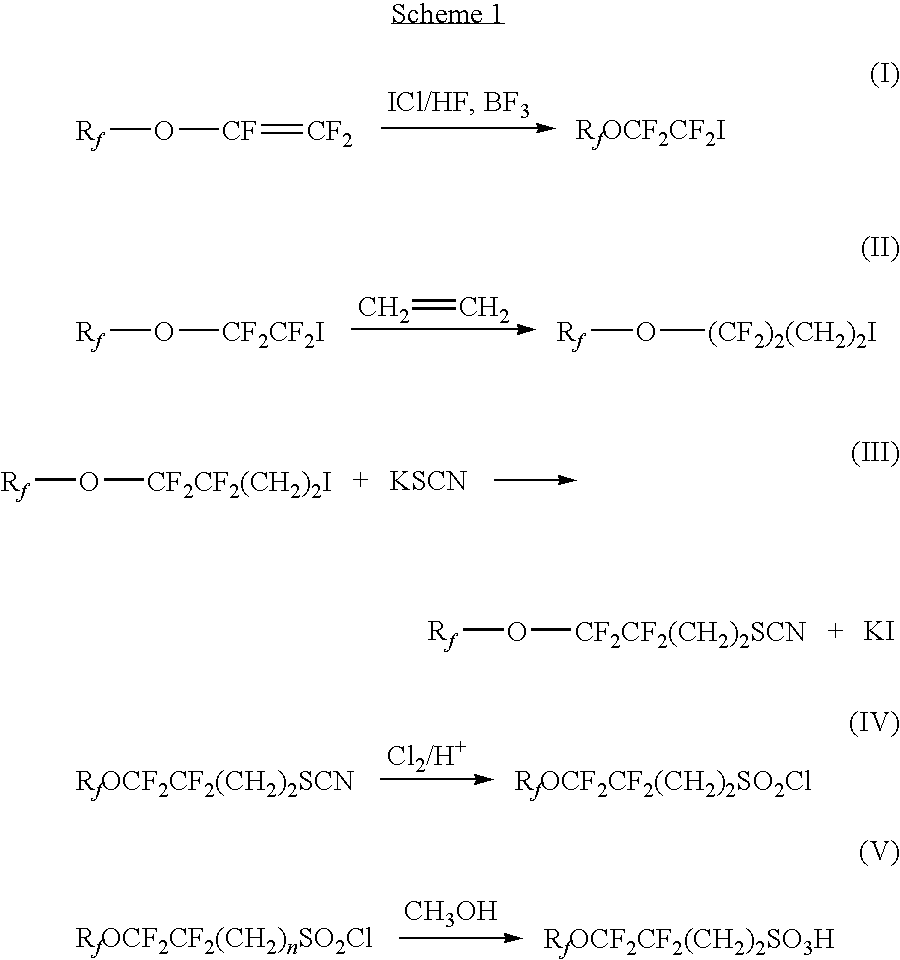
[0103]C3F7OCF2CF2I (100 g, 0.24 mol) and benzoyl peroxide (3 g) were charged to a pressure vessel under nitrogen. A series of three vacuum / nitrogen gas sequences was then executed at −50° C. and ethylene (18 g, 0.64 mol) was introduced. The vessel was heated for 24 hour at 110° C. The autoclave was cooled to 0° C. and opened after degassing. Then the product was collected in a bottle. The product was distilled giving 80 g of C3F7OCF2CF2CH2CH2I in 80% yield. The boiling point was 56˜60° C. at 25 mm Hg (3333 Pa).
[0104]Potassium thiocynate (21.34 g, 0.22 mol) was added to the mixture of C3F7OCF2CF2CH2CH2I (50 g, 0.11 mol) and trioctylmethylammonium chloride (0.2222 g) in 50 g of water. The reaction was heated overnight at 90° C. After phase separation, the product C3F7OCF2CF2CH2CH2SCN was distilled as a colorless liquid (32 g, 78%). The compound can be characterized by: b.p. 83˜85 oC / 2.3 torr; 1H NMR (CDCl3, 400 MHz) δ 3.10˜3.07 (2H, m), 2.58˜2.46 (2H, m); 19F NMR (CDCl3, 373 Hz) δ−81....
example 2
[0109]C3F7OCF2CF2I (100 g, 0.24 mol) and benzoyl peroxide (3 g) were charged to a pressure vessel under nitrogen. A series of three vacuum / nitrogen gas sequences was then executed at −50° C. and ethylene (18 g, 0.64 mol) was introduced. The vessel was heated for 24 hour at 110° C. The autoclave was cooled to 0° C. and opened after degassing. Then the product was collected in a bottle. The product was distilled giving 80 g of C3F7OCF2CF2CH2CH2I in 80% yield. The boiling point was 56˜60° C. at 25 mm Hg (3333 Pa).
[0110]C3F7OCF2CF2CH2CH2I (220 g, 0.5 mol) was added to the mixture of ethanol (250 mL) and water (250 mL). Sodium sulfite (126 g, 1 mol) was added, followed by 15 g copper. The reaction mixture was stirred vigorously under reflux for a week. 500 mL water was added and filtered at 75° C. The filtrate was cooled and the product C3F7OCF2CF2CH2CH2SO3Na was collected by filtration as white solid (86 g, 41.35%). The compound was characterized by: 1H NMR (CDCl3, 400 MHz) δ 3.19˜3.15 ...
example 3
[0115]Following the general procedure of Comparative Example B, the Surfactant Solution used in Example 3 was made from 0.88 g of C3F7OCF2CF2CH2CH2SO3Na dissolved in 500 mL of deionized water. There was no additional water precharge. Results are reported in Table 5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com