Compositions and Methods for Cancer Treatment
a technology of calcidiol and cancer, applied in the field of cancer chemotherapy, can solve the problems of severely limited clinical use of calcitriol and hampered treatment efficacy of systemically applied calcitriol analogs for cancer treatment, and achieve the effect of inhibiting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Analogs of B3CD
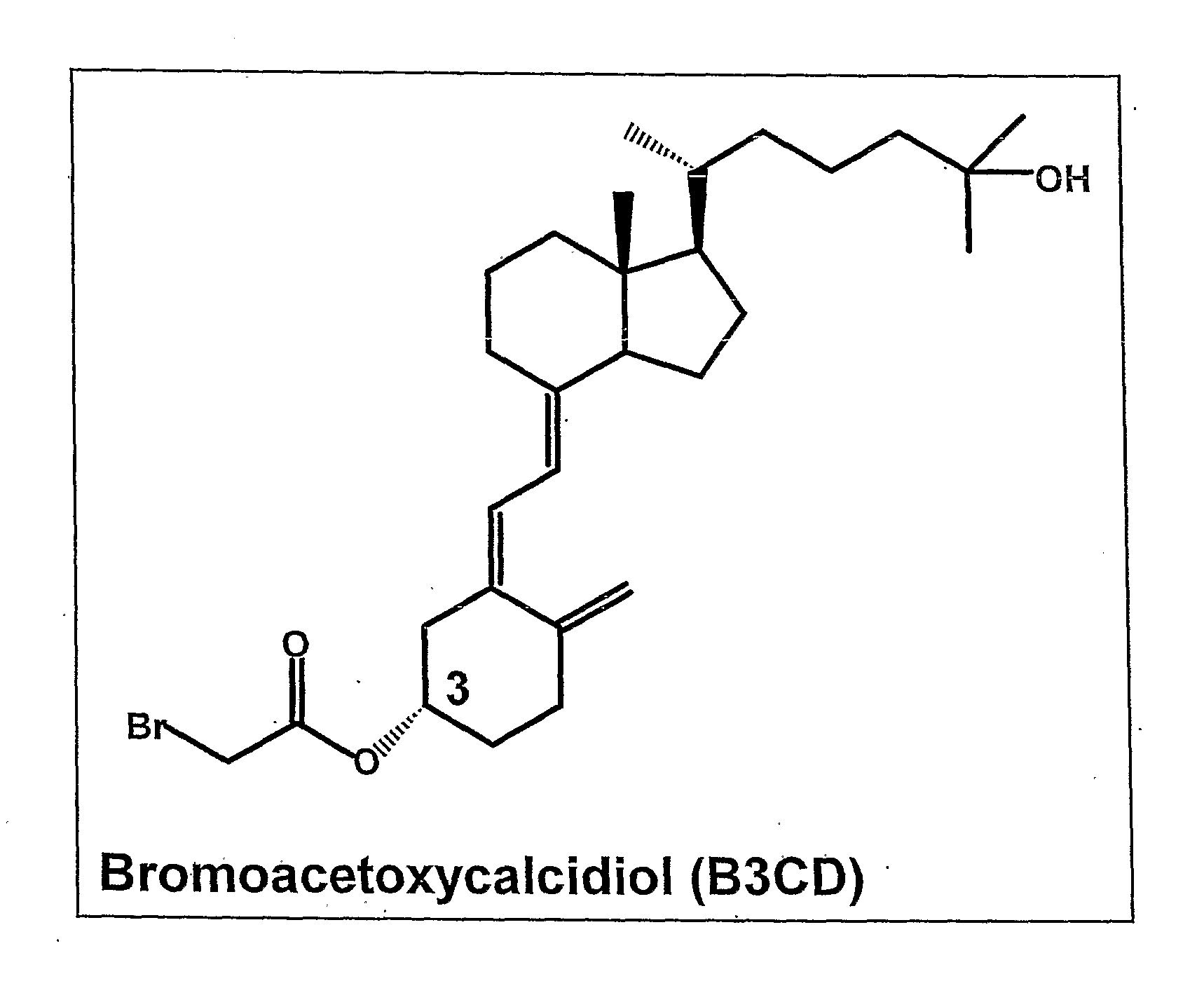
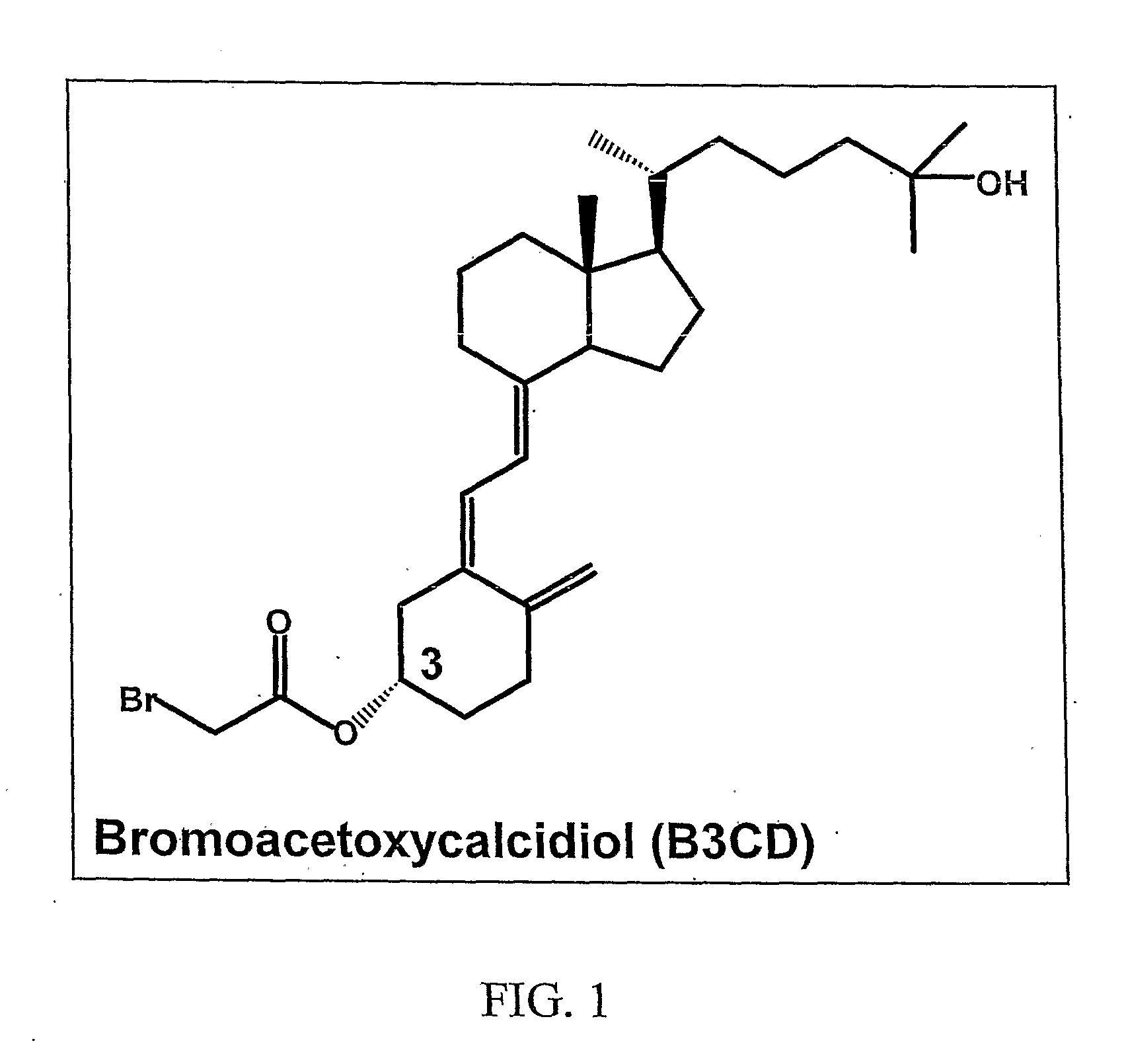
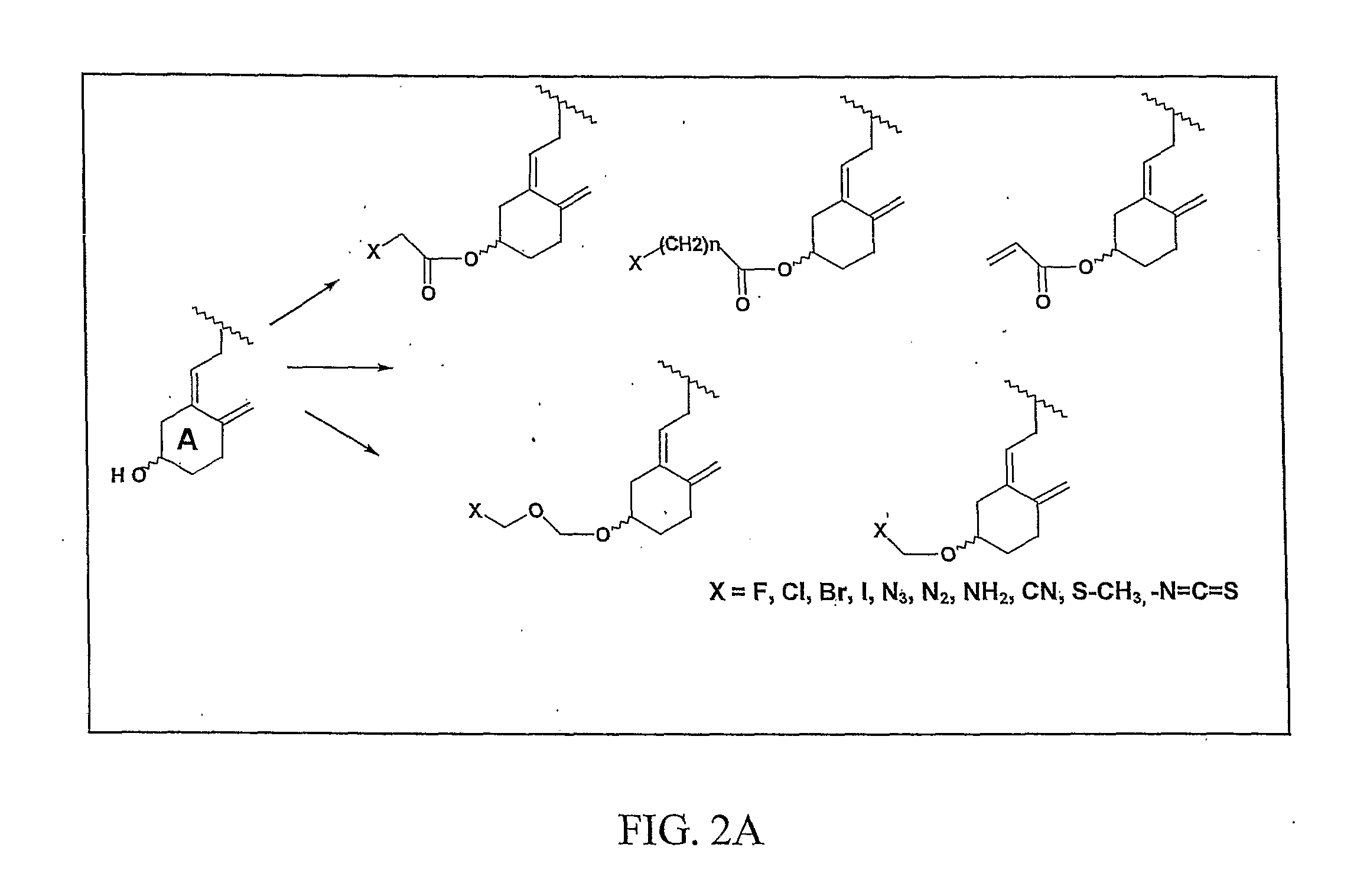
[0136]Derivatization of the A-ring hydroxyl group of calcidiol with bromoacetic acid imparted remarkable anticancer activity to B3CD. A library of compounds based on the B3CD scaffold will be designed and developed to improve the B3CD structure-activity profile by stepwise structural changes. A series of A-ring analogs of B3CD derivatized at the 3-OH position will be prepared (FIG. 2A). The basic A-ring synthon for these derivatives has been previously reported and readily incorporate electrophiles at C-3 position of A-ring (Frosch, J V, Lythogoe, B and Sakense, A K. Calciferal and its Derivatives. XVII. Ring A Components for synthetic work on vitamin D3 and on model compounds, J. Chem. Soc.: OrganieBioOrganic Chem 1974, 17:2005-2009). The important features of new derivatives will include replacement of —Br functionality with —F, —Cl, —Br, —I, —N3, —N2, —NH2, —CN, —S—CH3, and —N═C═S functionalities. The chain length between the A-ring and the functionality will be va...
example 2
Anticancer Activity
[0140]A panel of cancer cells is used to evaluate the antiproliferative and cytotoxic activities of B3CD and related analogs. The cells include colon (Colo), breast (MCF-7 and MDA-MB-231), lung (A549), prostate PC3 and LNCaP), melanoma (SK-MEL-31), renal (Caki-1), ovarian (OVCAR-3), bladder (T24), pancreatic (BxPC-3), hepatocarcinoma (Hep3B), neuroblastoma (SH-SY5Y), glioblastoma (SNB-19), medulloblastoma (D283 Med), skin (LS123), squamous cell carcinoma (Sec-15), acute promyelocytic leukemia (APL), myeloblastic (HL-60) and myelomonocytic (U937).
[0141]The anticancer activity testing will be carried out by high throughput screening. The cancer cells will be cultured in 96 well plates and treated with the library. B3CD has been tested in this system and was effective at a 200 nM dose. For general screening, 2-200 nM doses will be used. Cells will be treated with the compounds for 12, 24, 48 and 72 hours. A 96 well plate format allows testing a large number of compou...
example 3
In Vitro Treatment of Neuroblastoma Cells
[0143]Materials and methods. Proliferation was measured by MTS and BrdU incorporation assays. Apoptosis was assessed by DNA fragmentation, Cell Death Detection ELISA and caspase-3-assays. Effect of B3CD on TrkB signaling by BDNF was assessed by Western Blot analysis of AKT and ERK phosphorylation. The effect on angiogenesis was determined by chick chorioallantoic membrane (CAM) assay and aortic ring assay.
[0144]B3CD was tested on the panel of cell described above. B3CD showed potent antiproliferative and cytotoxic effects on neuroblastic, ovarian, endothelial cells and prostate cancer cells, but did not show an effect on proliferation of skin, smooth muscle, breast, pancreatic, osteosarcoma and macrophage cells (FIG. 3). Such cell-type specificity is not uncommon. In addition, B3CD induced apoptosis; distinct DNA laddering characteristic of apoptosis was observed in neuroblastic, ovarian and prostate cancer cells. However, keratinocytes were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com