Helicobacter pylori bacterium proliferation inhibitor
a technology of helicobacter pylori and proliferation inhibitor, which is applied in the direction of antibacterial agents, drug compositions, sugar derivates, etc., can solve the problems of large manufacturing facilities, inability to synthesize cgl by itself, and high cost, so as to facilitate the curing or alleviating of peptic diseases and improve the h
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1.1 Chemical Synthesis of GlcNAc1-beta-O-Et (2)
[0039]Ethoxy 2-acetamide-2-deoxy-N-acetyl-beta-D-glucosaminide (GlcNAc1-beta-O-Et (2)) as an example of the N-acetylglucosaminyl beta-linked monosaccharide derivative represented by the foregoing chemical formula (1) to which the present invention is applied is detailed in this Example 1. This derivative can be synthesized according to the following chemical reaction scheme (3).
[0040]Specifically, 3.0131 g (13.62 mmol) of N-acetyl-D-glucosamine was added to a 200 mL volume eggplant-shaped flask containing HCl gas-bubbled EtOH (50.0 mL) to thus dissolve the compound into the latter, a tube packed with calcium chloride was attached to the flask and the resulting solution was then stirred at room temperature. Whether the reaction was completed or not was confirmed by the thin layer chromatography (TLC) technique (developer solvent: chloroform / methanol (3:1)). After 17 hours, NaHCO3 was added into the reaction mixture to neutralize and then...
example 2
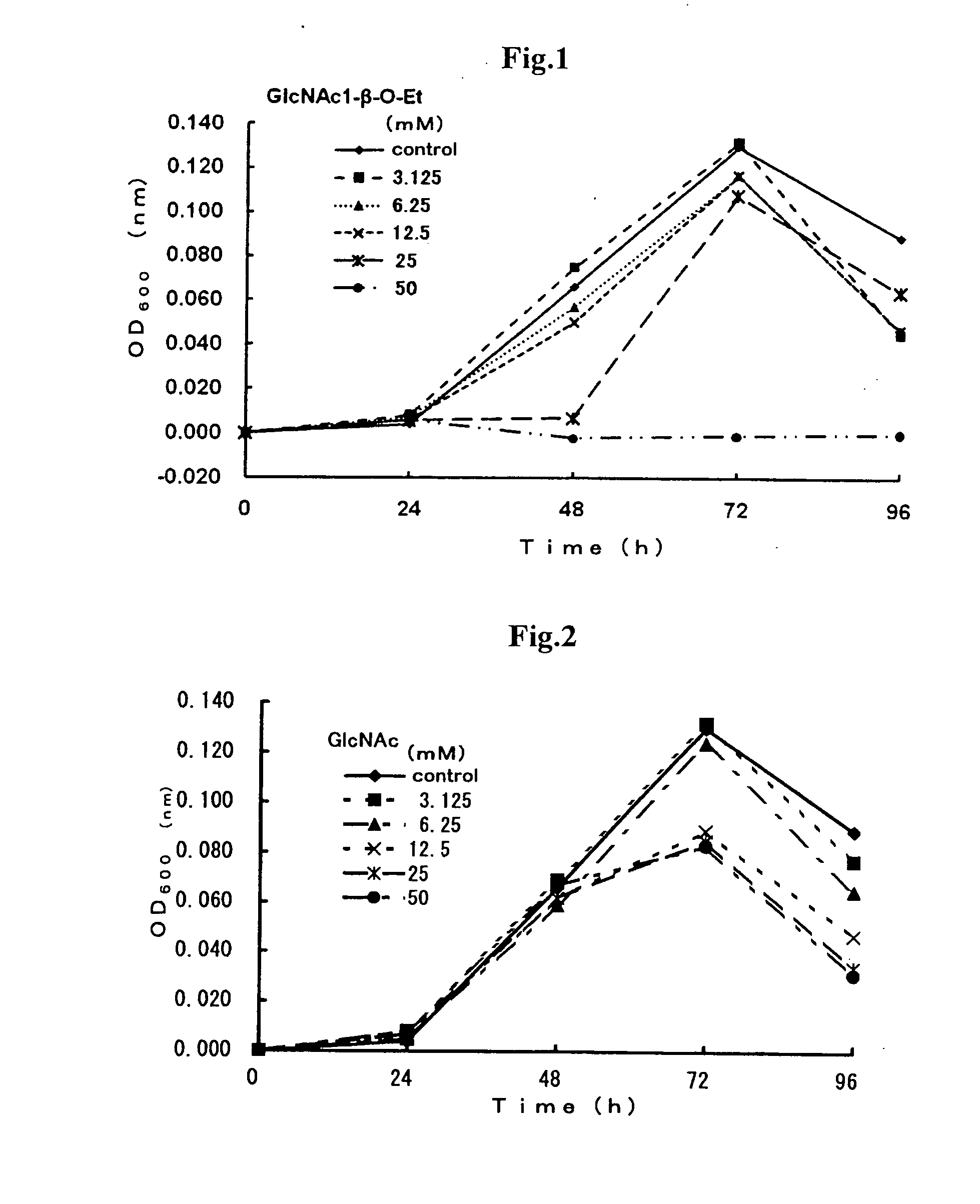
2.1 Preparation of Proliferation Inhibitor of H. pylori Bacteria Comprising GlcNAc1-beta-O-Et (2) and Confirmation of H. pylori Bacteria-Proliferation Inhibitory Effect Under In Vitro
[0046]The effect of GlcNAc1-beta-O-Et (2) on H. pylori bacteria was confirmed according to the following procedures. Bacterial cells of H. pylori bacteria (ATCC 43504) stored in a brucella broth culture medium frozen at −80° C. were cultured in the same culture medium (3 mL) supplemented with 10% horse serum at 35° C. in the presence of 15% CO2 for 40 hours according to the shaking culture technique, the movement or behavior of bacterial cells was observed under a microscope and non-coccoid type bacterial cells of H. pylori bacteria were recovered. The culture medium was inspected for the optical density (OD) values at 600 nm, followed by the dilution thereof with Muller-Hinton broth culture medium supplemented with 5.5% horse serum such that the number of bacterial cells present therein was equal to 4×...
example 3
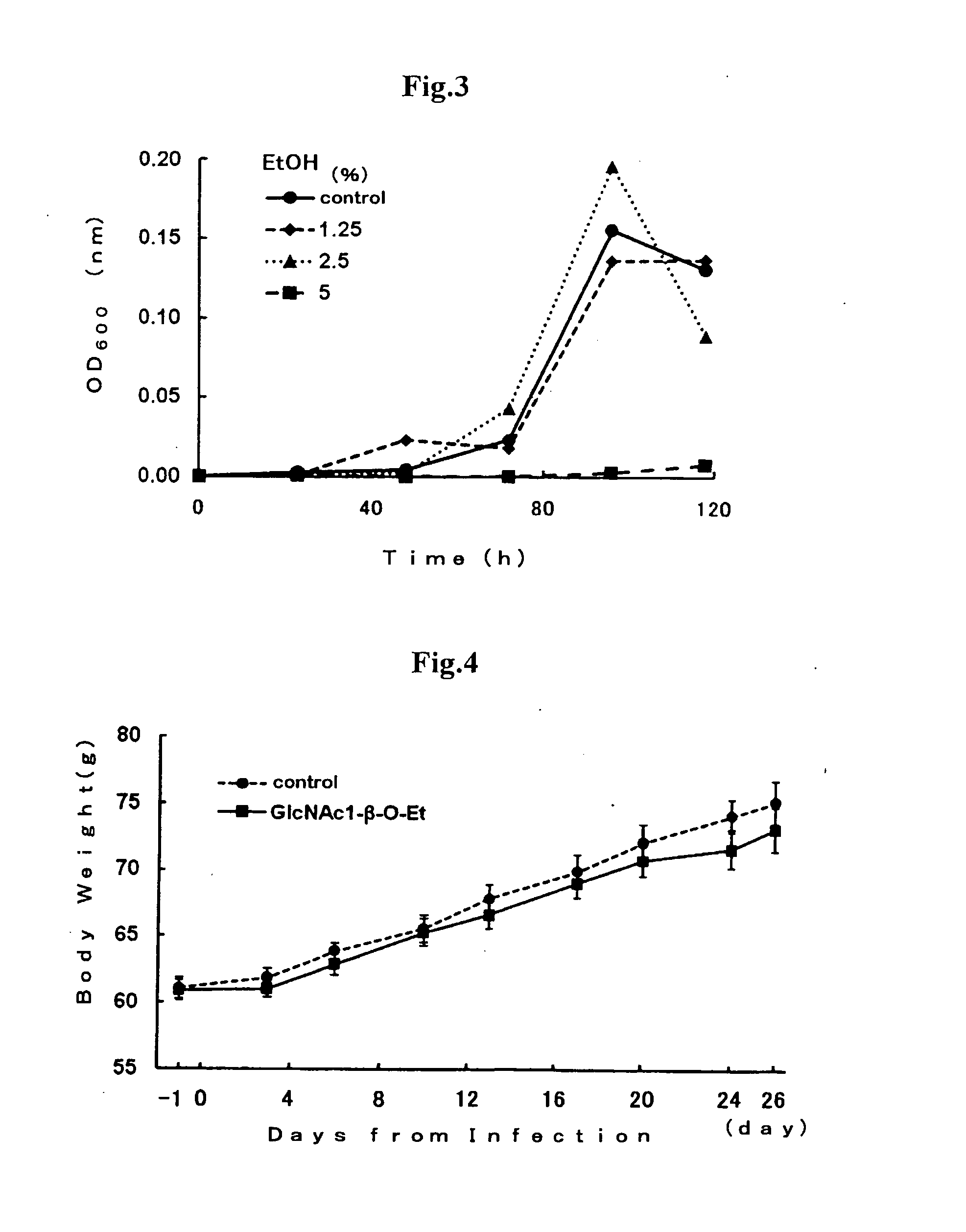
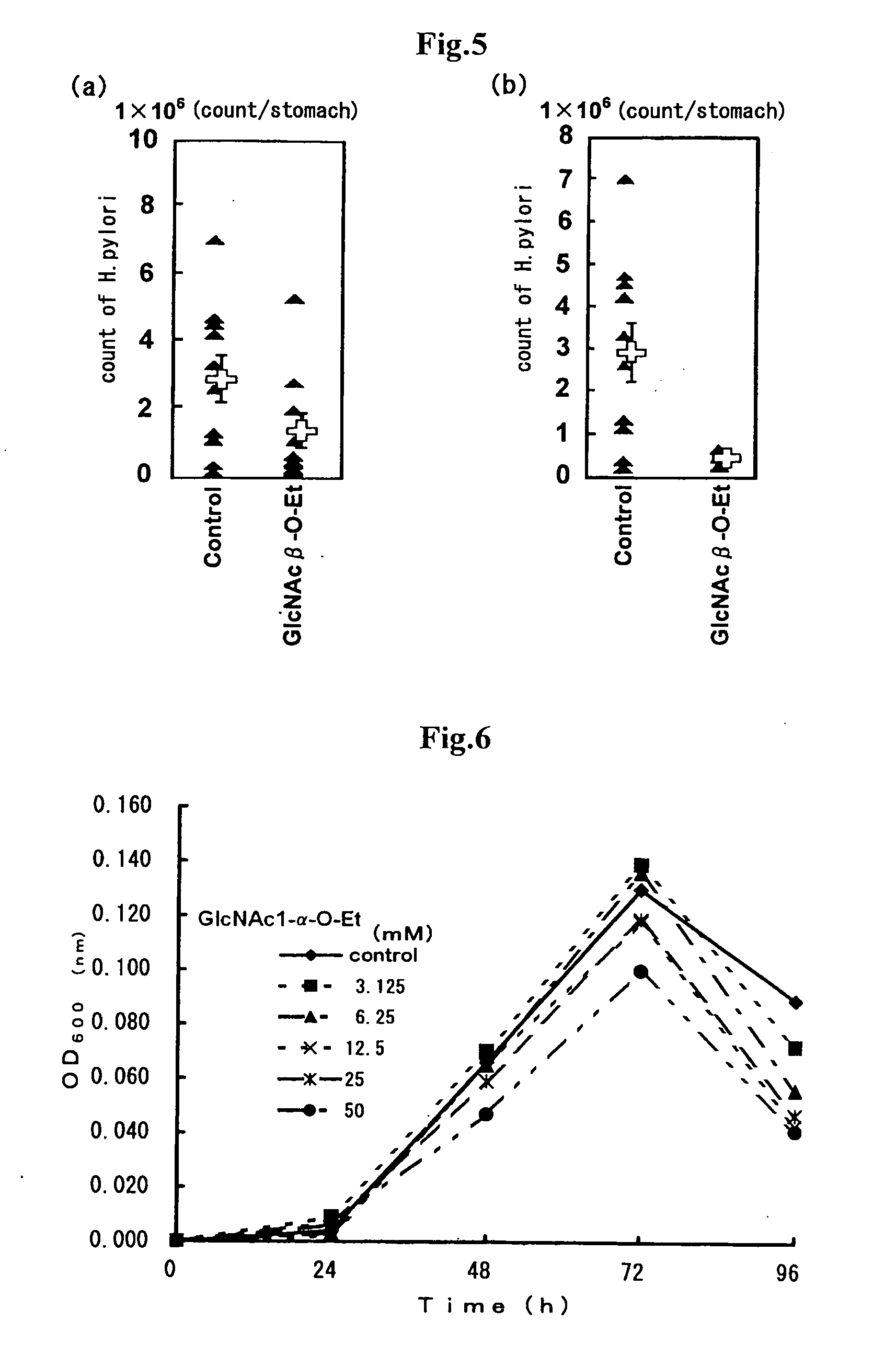
3.1 Preparation of Proliferation Inhibitor of Helicobacter pylori Bacteria Comprising GlcNAc1-beta-O-Et (2) and Confirmation of H. pylori Bacteria-Proliferation Inhibitory Effect Under In Vivo
[0054]Antibacterial activities of GlcNAc derivatives towards H. pylori bacteria were investigated under in vivo by an experimental system using meriones unguiclatus infected orally with H. pylori bacteria.
3.2(1) Animal for the Experiments
[0055]4 weeks-old male meriones unguiculatus (SPF: specific-pathogen free), which were purchased from Kyudo Co., Ltd. (Br. Yoshitomi), were preliminarily reared for 23 days and then used for the experiments. The meriones unguiculatus were reared temperature of 24 plus or minus 3° C. and under relative humidity of 55 plus or minus 15% in all room for infected animals with conditions of lighting from a.m. 7 to p.m. 7 and ventilating 18 times per hour during preliminarily reared period and experiment period. 2 or 3 meriones unguiculatus were reared in a cage. All ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com