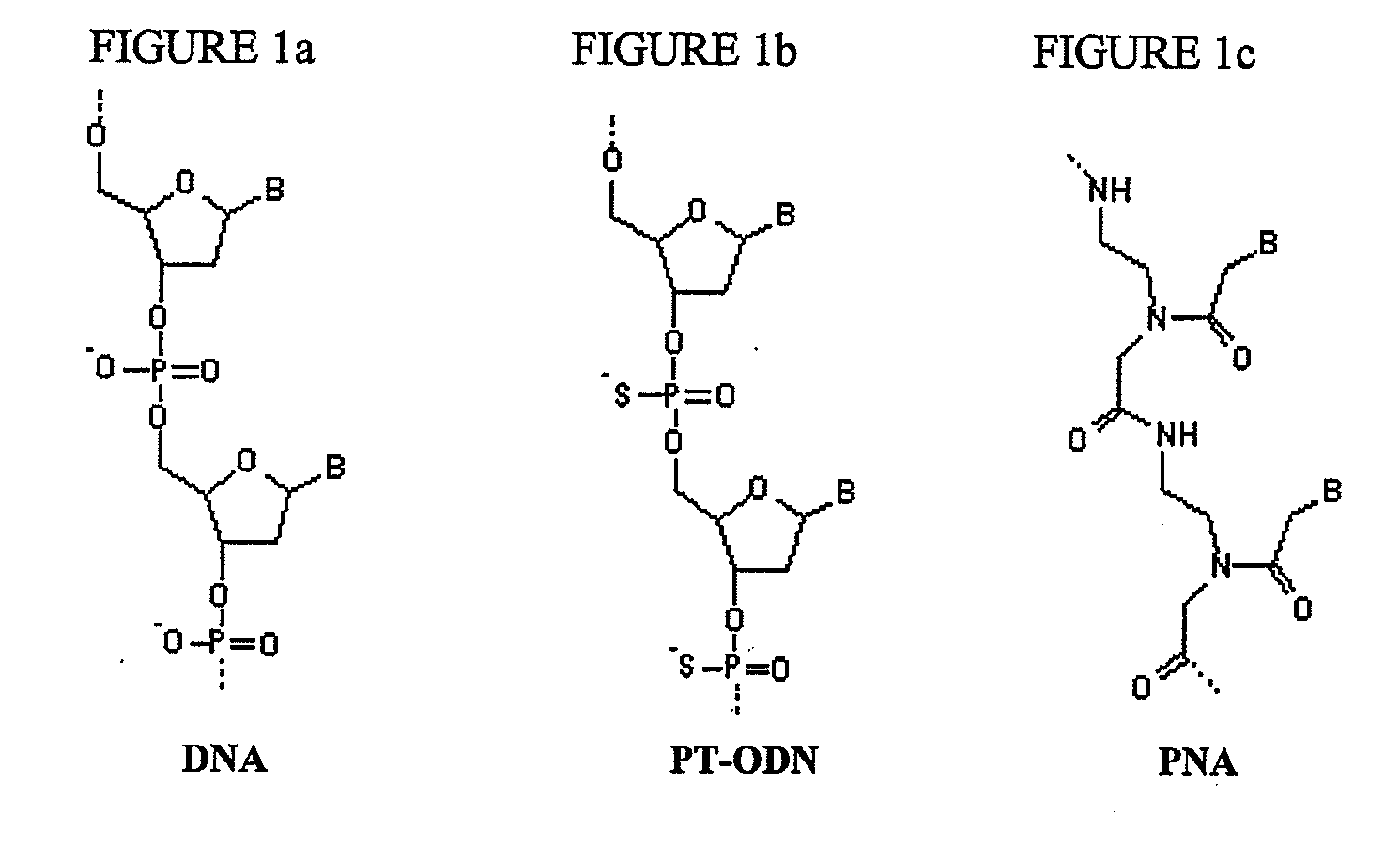
Nanoparticles containing plymeric nucleic acid homologs, pharmaceutical composition and articles of manufacture containing same and methods of use thereof
a technology of nucleic acid homologs and nanoparticles, which is applied in the direction of powder delivery, microcapsules, tissue culture, etc., can solve the problems of cationic liposomes, immune response and/or oncogenesis, and less effective than real viruses in dna delivery capacity, etc., and achieve high efficiency and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparative Formulation of PLGA-pDNA
[0173]In order to determine an optimum formulation for PLGA-pDNA nanospheres, various calcium concentrations and calcium to pDNA ratios were employed. Results of physicochemical characterization of the formulations are summarized in Table 1 (presented hereinabove). The addition of calcium to the exterior phase (formulations NP1-NP3) resulted in a high encapsulation efficiency of about 70% in comparison with only 28% in the absence of calcium (NP0). Increasing the initial concentration by two-fold, or increasing the calcium / DNA ratio by two-fold resulted in insignificant changes in the final loading of pDNA in the NP. pDNA in exterior and pDNA entrapped (Table 1, rows 3 and 4) do not sum to 100% since some pDNA was apparently lost in the interphase between chloroform and aqueous phase during extraction. Reduction of 33% in calcium concentration was found in the exterior phase. However, the same reduction in calcium concentration was found in both p...
example 2
Analysis of Structural and Functional Integrity of pDNA Encapsulated in PLGA Nanoparticles
[0175]Plasmid DNA Encapsulated in PLGA NP retained its structural integrity following enzymatic digestion and exhibited a pattern similar to intact pDNA (FIG. 4b). However, the ratio between relaxed (nicked) to supercoiled conformation of encapsulated pDNA in comparison with control pDNA increased from 30:70 to 60:40% (FIG. 4a). pDNA either released or extracted from the various formulations (Table 1; presented hereinabove in methods and materials) had a similar relaxed / supercoiled pattern, and was found to be bioactive as indicated by its transfection efficiency in NIH 3T3 cells (Table 2). The decreased proportion of supercoiled DNA was loosely correlated to lower expression levels but the difference was statistically insignificant. AP expression level of naked pDNA was 17.1×105±10.4×105. No statistical difference was found between the various groups. These results indicate that that the addit...
example 3
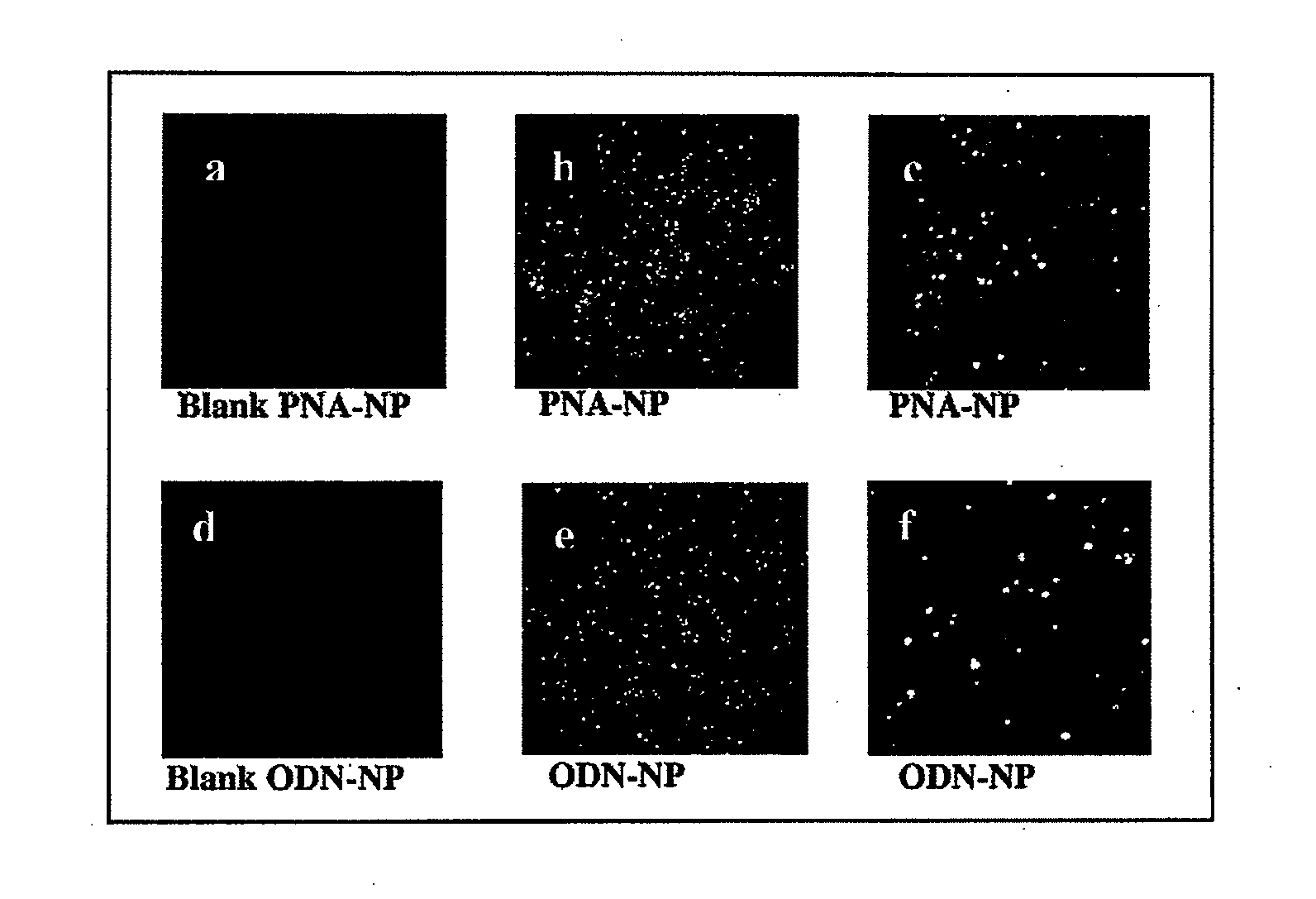
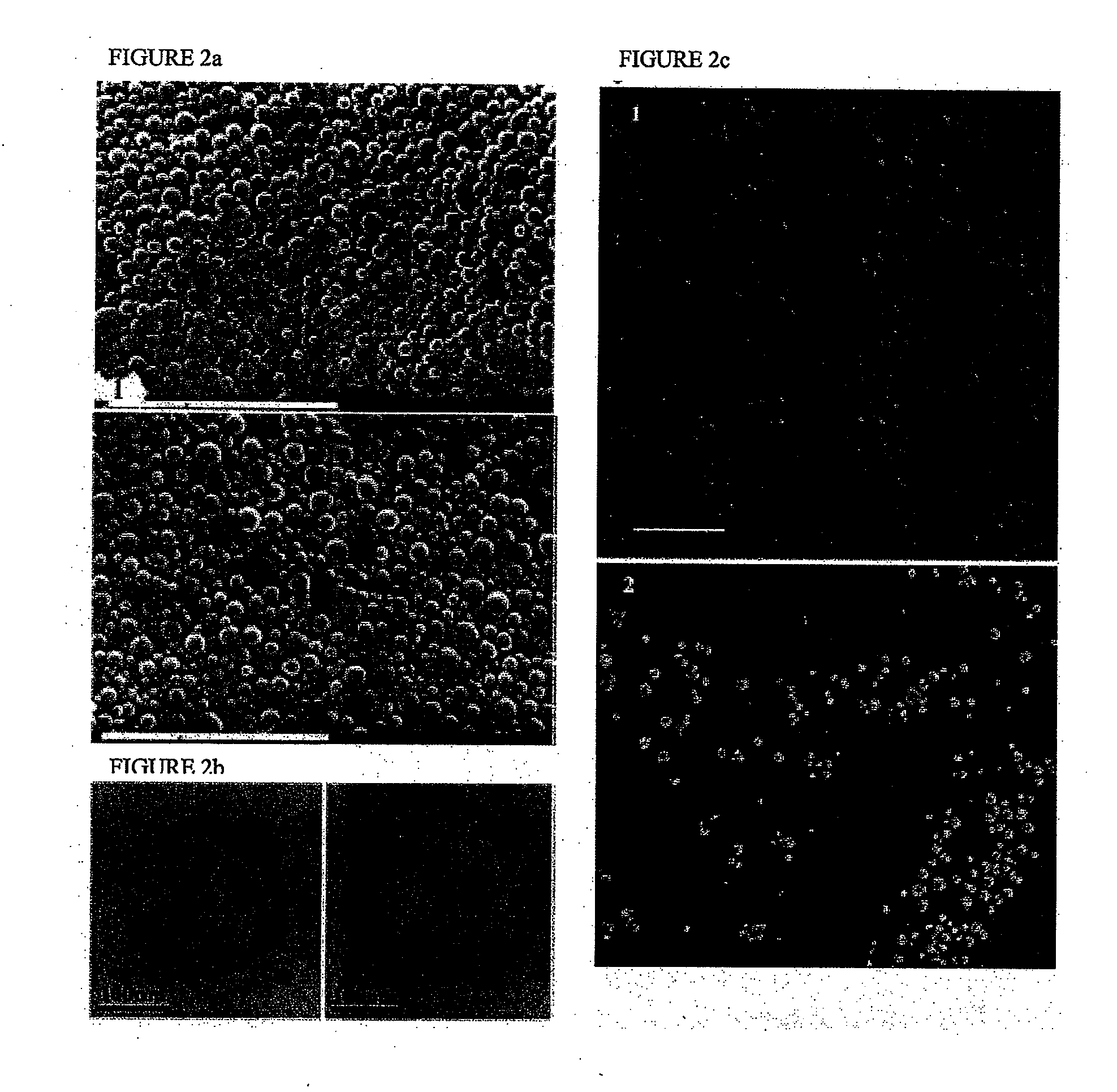
Cellular Incorporation of ODN Encapsulated in PLGA Nanoparticles
[0176]In light of the results described in example 2, an examination of the cellular localization of nanoparticles within cells was conducted. Empty nanoparticles prepared from pyrene-PLGA polymer were incorporated into cells and accumulated in the cytoplasm of transfected NIH 3T3 cells (FIG. 5). In order to examine the cellular uptake of pDNA-NP, encapsulated pDNA was labeled with the fluorescent probe, TOTO-1. This cell-impermeable fluorescent probe exhibits large fluorescence enhancement (1400-fold) upon binding to pDNA. Both blank-NP and naked plasmid exhibited very low fluorescence, determined as background for further analyses. In contrast, both pDNA-NP and released pDNA were detected inside the cells (FIG. 6a-c). pDNA-NP were concentrated mainly in the cytoplasm (FIGS. 6a and c). In contrast, released pDNA (morphology resembling a nanosphere is not observed) was detected in the cytoplasm (FIG. 6a), around the nuc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com