Fluoroolefin/vinyl alcohol copolymer and process for its production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0080]Polymerization Step:
[0081]Into a stainless steel autoclave having an internal capacity of 200 mL and equipped with a stirrer (pressure resistance: 3 MPa), 79.0 g of t-butyl alcohol, 26.7 g of t-butyl vinyl ether (hereinafter referred to as “TBVE”) as the vinyl ether (b), 0.48 g of potassium carbonate and 0.46 g of an isooctane solution containing 70% of perbutyl perpivarate (hereinafter referred to as “PBPV”) were charged, and the oxygen in the system was removed by repeating pressure purging with N2 gas. Then, 26.7 g of tetrafluoroethylene (hereinafter referred to as “TFE”) as the fluoroolefin (a) was introduced into the autoclave, followed by heating to 55° C. The pressure at that time was 1.56 MPa. Then, polymerization was continued for 7 hours, and when the pressure decreased to 1.12 MPa, the autoclave was cooled with water, and non-reacted TFE was purged to terminate the polymerization. The obtained polymer solution was put into methanol to precipitate the formed copolyme...
example 2
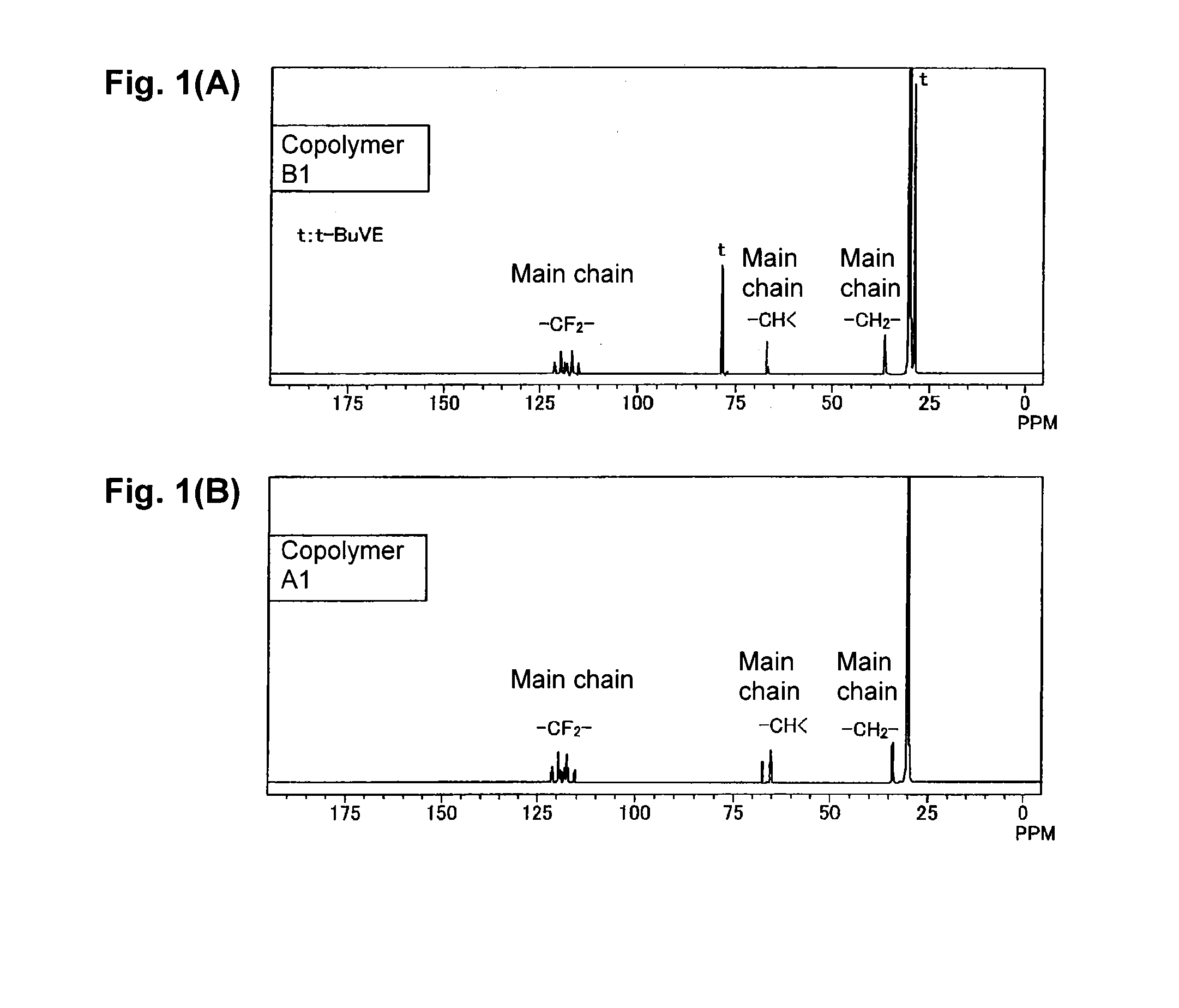
[0086]The copolymer B1 obtained in Example 1 was used.
[0087]Deprotection Step:
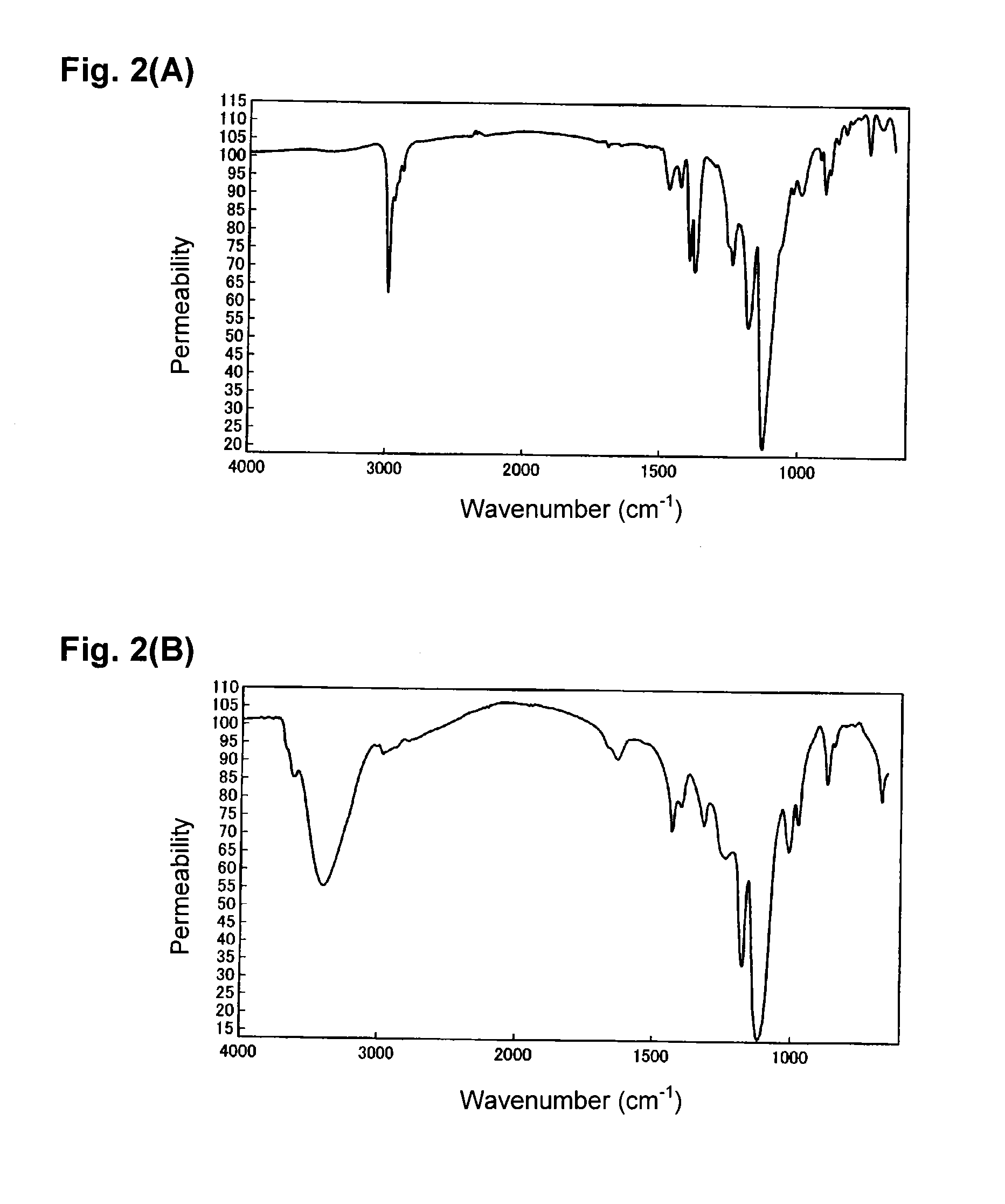
[0088]2.0 g of the copolymer B1, 50 mL of 4N hydrochloric acid and 1 mL of 1,4-dioxane were put into a 100 mL flask, heated and stirred at 90° C. to carry out a deprotection reaction. This reaction system gradually became a uniform solution. The reaction was continued for a total of 12 hours, and then, the reaction solution was dropped into water to precipitate copolymer A2, which was washed with water and then vacuum-dried at 40° C. to isolate 1.49 g of the copolymer A2.
[0089]By the measurements of the 1H-NMR spectrum and the IR spectrum, it was confirmed that in the copolymer A2, at least 97% of protective groups (t-butyl groups) were eliminated.
example 3
[0090]The copolymer B1 obtained in Example 1 was used.
[0091]Deprotection Step:
[0092]2.0 g of the copolymer B1, 50 mL of trifluoroacetic acid and 1 mL of methylene chloride were put into a 100 mL flask and then stirred at room temperature. The reaction was continued for a total of 48 hours, and then, the precipitated copolymer was washed with water and then vacuum-dried at 40° C. to isolate 1.33 g of the copolymer A3.
[0093]By the measurements of the 1H-NMR spectrum and the IR spectrum, it was confirmed that in the copolymer A3, at least 97% of protective groups (t-butyl groups) were detached.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com