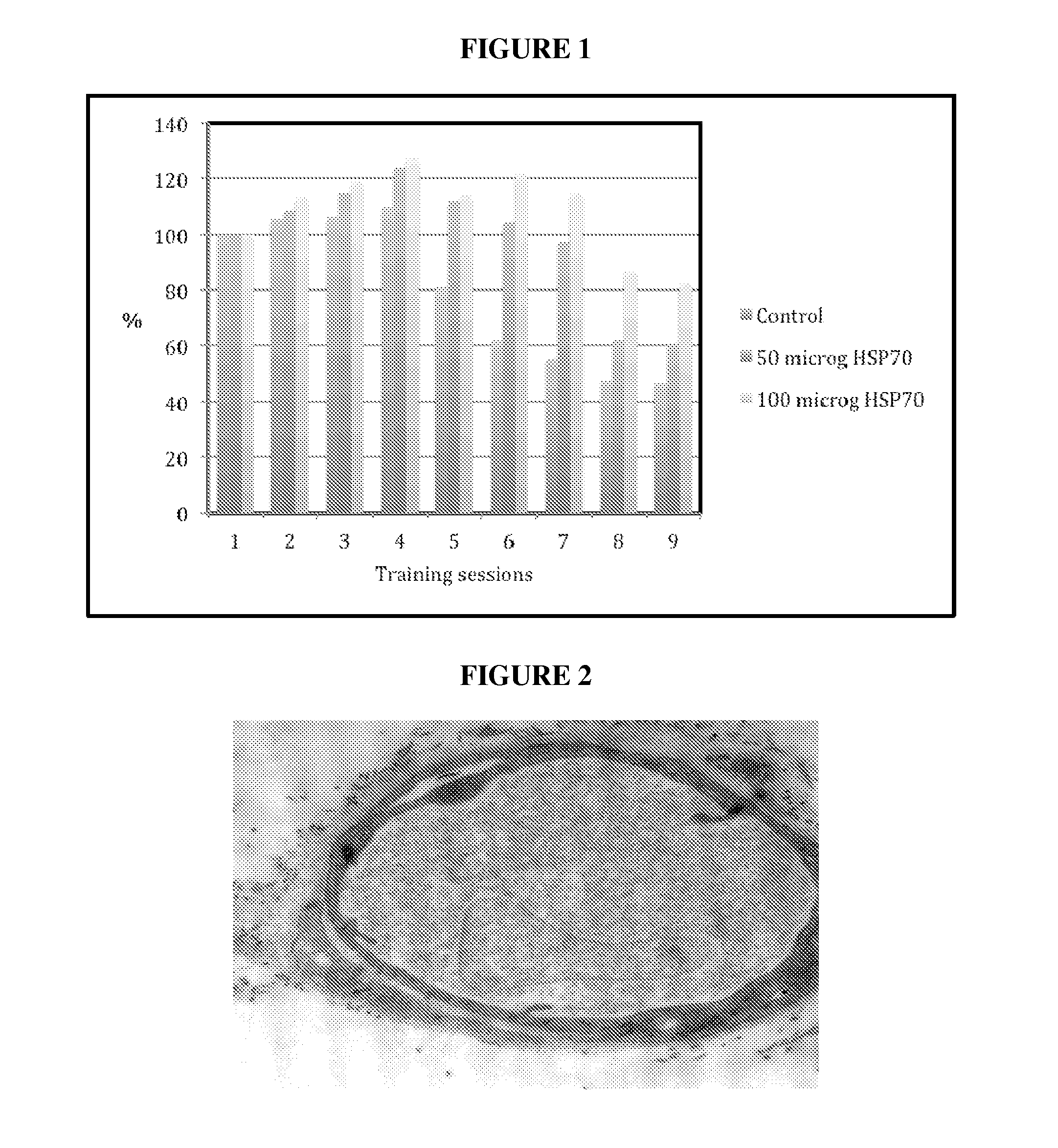
Novel methods of use of hsp70 for increased performance or treatment of hsp70 related disorders
a technology of hsp70 and hsp70, which is applied in the direction of peptide/protein ingredients, drug compositions, muscular disorders, etc., can solve the problems of limited approach, limited key therapeutic approaches and care standards, and the tens of billions of dollars associated with treatment and care of musculoskeletal diseases. achieve the effect of fatigue and increase performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fermentation of rhHSP70
[0082]To prepare sufficient amounts of HSP70, 5 L of the E. coli expression strain HMS 174 (DE3) hHSP70 may be grown in animal free medium containing 12 g phytone peptone, 60 g yeast extract, 40 g sodium chloride, 1g methionine, and water to 5 liters. Before inoculation with expression strain bacteria, the medium may be sterilized, and supplemented with kanamycin to a final concentration of 30 mcg per milliliter. The medium may then be inoculated with one liter of an overnight culture of the HMS174(DE3) bacteria. The pH, level of dissolved oxygen and temperature inside the fermenter reactor may be carefully monitored. When the culture reaches an optical density of approximately 1.2 (for example, approximately 2.5 hours after inoculation), IPTG (isopropyl-B-D-thiogalactopyranoside) may be added to a final concentration of 1 mM to initiate the induction of expression of rhHSP70. Growth may then be continued until an optical density of approximately 2.7 is attain...
example 2
Purification of rhHSP70
[0083]A bacterial lysate comprising rhHSP70 may be subjected to the following two-step purification protocol. The specific example provided utilizes 500 mL of lysate; adjustments may be made for different lysate volumes. In the first step of the protocol, 500 mL of the cleared lysate, prepared as set forth above, may be diluted two-fold with 10 mM sodium phosphate buffer pH 7.0. This 1 L of diluted and cleared bacterial lysate may be loaded onto a of DEAE Sephacel (Pharmacia) column (13 cmx 15 cm) previously equilibrated with 2 column volumes of buffer A (10 mM sodium phosphate buffer pH 7.0, 20 mM sodium chloride, 10 mM ammonium sulfate). The elution is carried out with two column volumes of buffer B (20 mM sodium phosphate buffer pH 7, 85 mM sodium chloride, 10 mM ammonium sulfate). About 2 L of eluate is expected for recovery. The eluate may then be diafiltered (buffer exchanged) against a 10-fold volume of buffer C (20 mM sodium citrate pH 6.0, 100 mM sodi...
example 3
PEGylation Techniques
[0085]The efficacy of a therapeutic agent may be enhanced by improving its bioavailability via several means, one of which is PEGylation, a process of chemically linking polyethylene glycol (PEG) to the therapeutic agent of interest, with the resulting conjugate exhibiting an increased serum half-life. Additional advantages of the PEGylated products may also include lower immunogenicity, decreased dosing frequency, increased solubility, enhanced stability, and reduced renal clearance. Because the most common reactive sites on proteins (including peptides) for attaching PEG are the c amino groups of lysine and the a amino group of the N-terminal residue, early methods of PEGylation resulted in modification of multiple sites, yielding not only monoPEGylated conjugates consisting of mixtures of positional isomers, such as PEGINTRONTM (Grace et al., J. Biol. Chem. 2005; 280:6327) and PEGASYS® (Dhalluin et al., Bioconjugate Chem. 2005; 16:504), but also adducts compr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com