Thermostable Vaccine Compositions and Methods of Preparing Same
a technology of vaccine compositions and thermostables, applied in the field of thermostable vaccine compositions, can solve the problems of significant limitations in the use of aluminum-salt adjuvants in many subunit vaccines based on recombinant, the influence of vaccine storage stability, and the relative weakness of aluminum adjuvants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
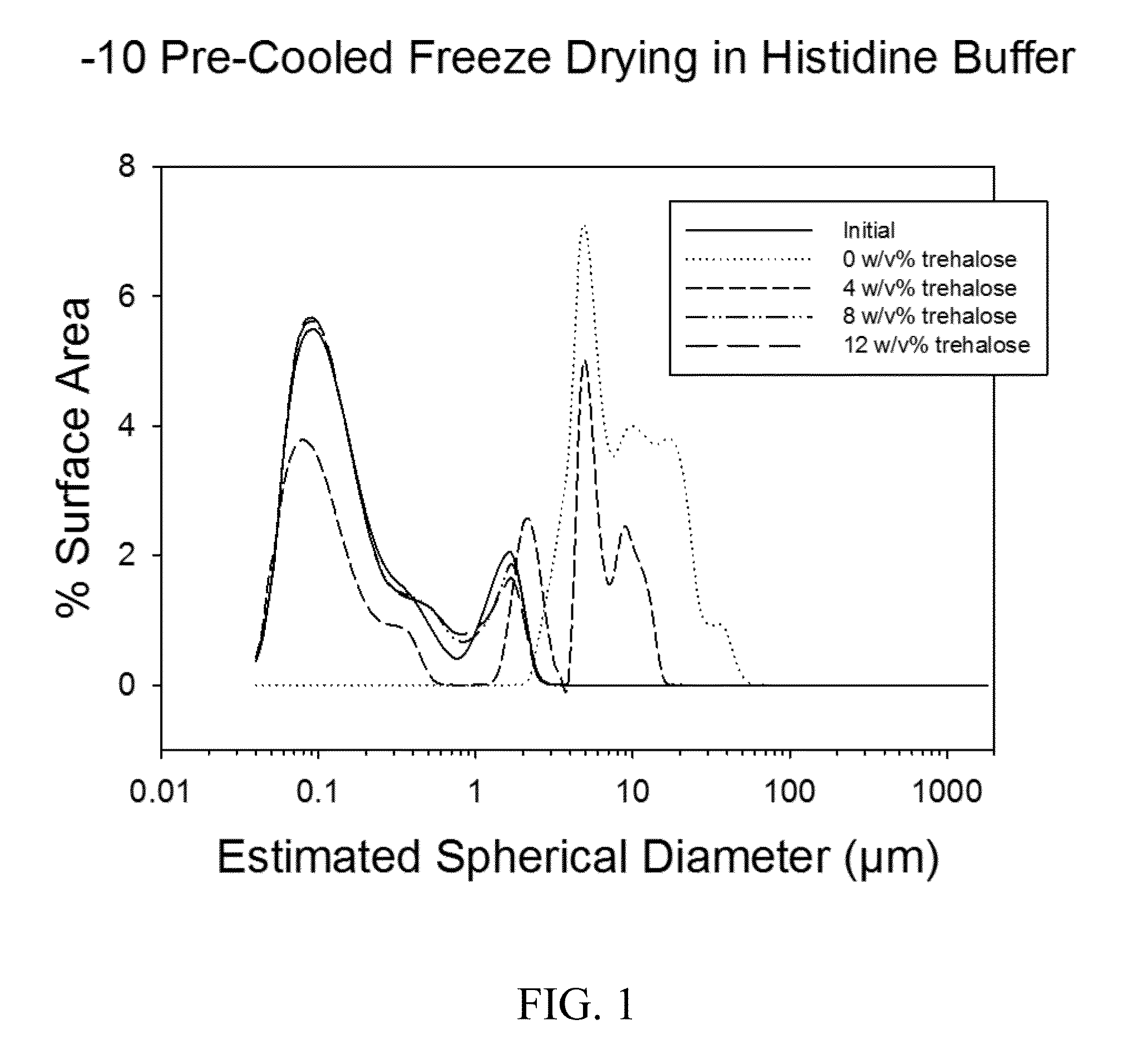
I. −10° C. Pre-Cooled Tray Freeze Drying with Varying Settling Time of Particles
[0054]10 mM histidine buffer at pH 6.0, 1 mg / mL Al from Alhydrogel, and 0, 4, 8 or 12 w / v % trehalose was combined and rotated end over end for 30 minutes at 4° C. 1 mL of the solution was placed in each 3 mL glass freeze drying vial. The formulations were placed on −10° C. pre-cooled shelves in the freeze drier and freeze dried as follows in the table below. Following freeze drying, the chamber was backfilled with dry nitrogen gas and the vials were sealed.
TABLE 1InitialFinalTempTempStageTime for Step(° C.)(° C.)PressureRateFreezing0.25hours−10−10AtmosphericConstanttemp −101hours−10−40Atmospheric−0.5° C. / min1hour−40−40AtmosphericConstanttemp −40Primary0.5hours−40−4060 mTorrConstantDryingtemp −400.5hour−40−2060 mTorrIncreasetemp20hours−20−2060 mTorrConstanttemp −20Secondary1 hour 40 min−20060 mTorr0.2° C. / Dryingmin1hour03060 mTorr0.5° C. / min5hours303060 mTorrConstanttemp 30
Particle Size Analysis
[0055]Par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com