Formation of layers of amphiphilic molecules
a technology of amphiphilic molecules and amphiphilic ions, which is applied in the field of amphiphilic molecule layer formation, can solve the problems of difficult scaling, laborious and time-consuming laboratory methods, and difficult to overcome conditions and expertise required to achieve the effect of reducing the amount of hydrophobic fluid, reducing the sensitivity of the apparatus, and reducing the amount of excess fluid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
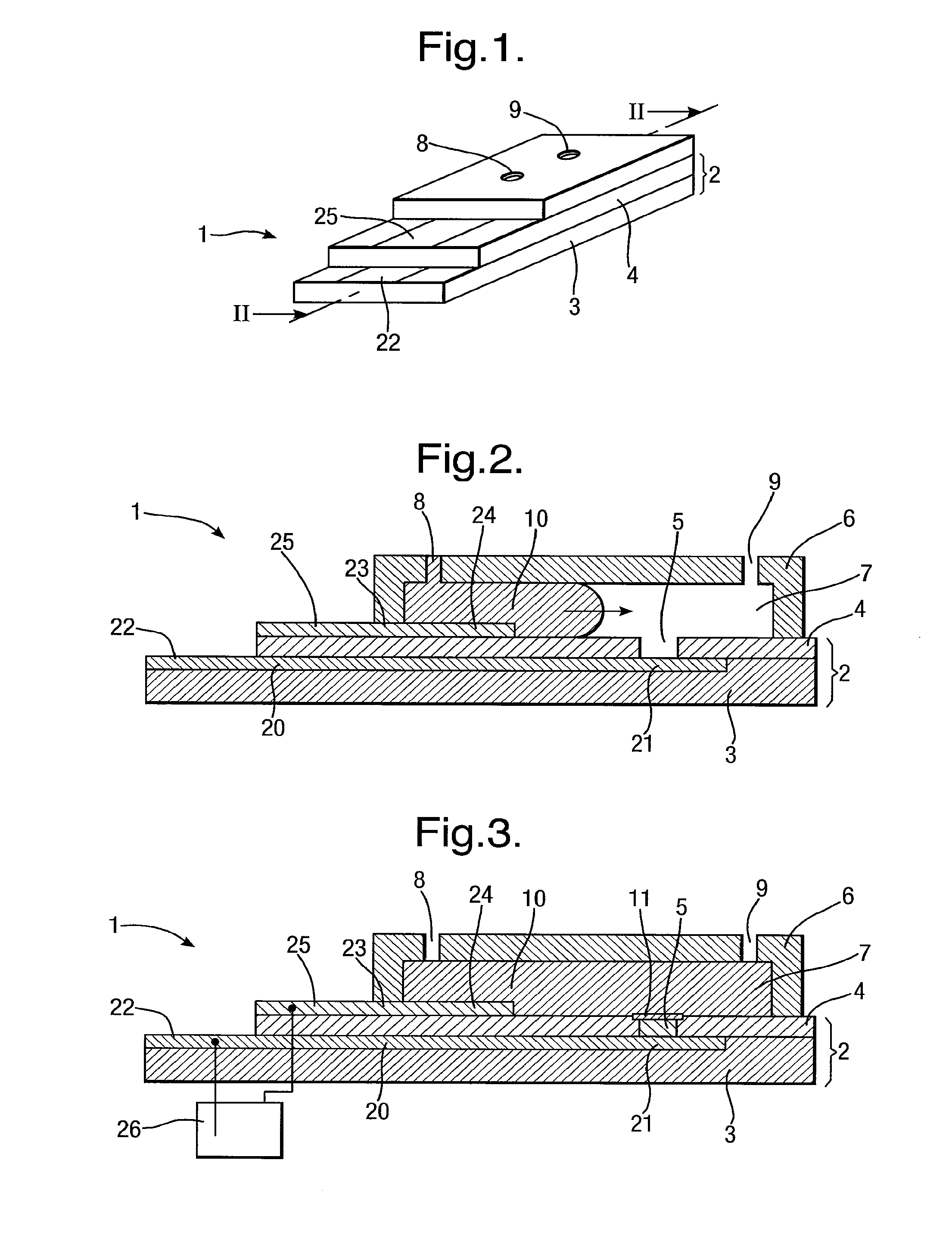
[0208]The apparatus 1 described above has been made and used experimentally to demonstrate formation of a layer 11, in particular being a lipid bilayer, and insertion of a membrane protein, for examplem α-hemolysin. The following procedure was followed after manufacture of the apparatus 1:
[0209]1) apply pre-treatment coating 30 to body 2;
[0210]2) introduce aqueous solution 10 into chamber 7 to cover recess 5;
[0211]3) electro-wet the electrode 21;
[0212]4) remove aqueous solution 10 to un-cover recess 5 and re introduce aqueous solution 10 into chamber 7 to cover recess 5 and form the layer 11;
[0213]5) add α-hemolysin free into aqueous solution 10 and monitor insertion into layer 11.
[0214]In step 1), the pre-treatment coating 30 was hexadecane dissolved in pentane. The quantity and volume of the pre-treatment coating 30 was varied for each test to obtain the optimum conditions for formation of the layer 11. Insufficient pre-treatment coating 30 prevented formation of the layer 11 whil...
example 2
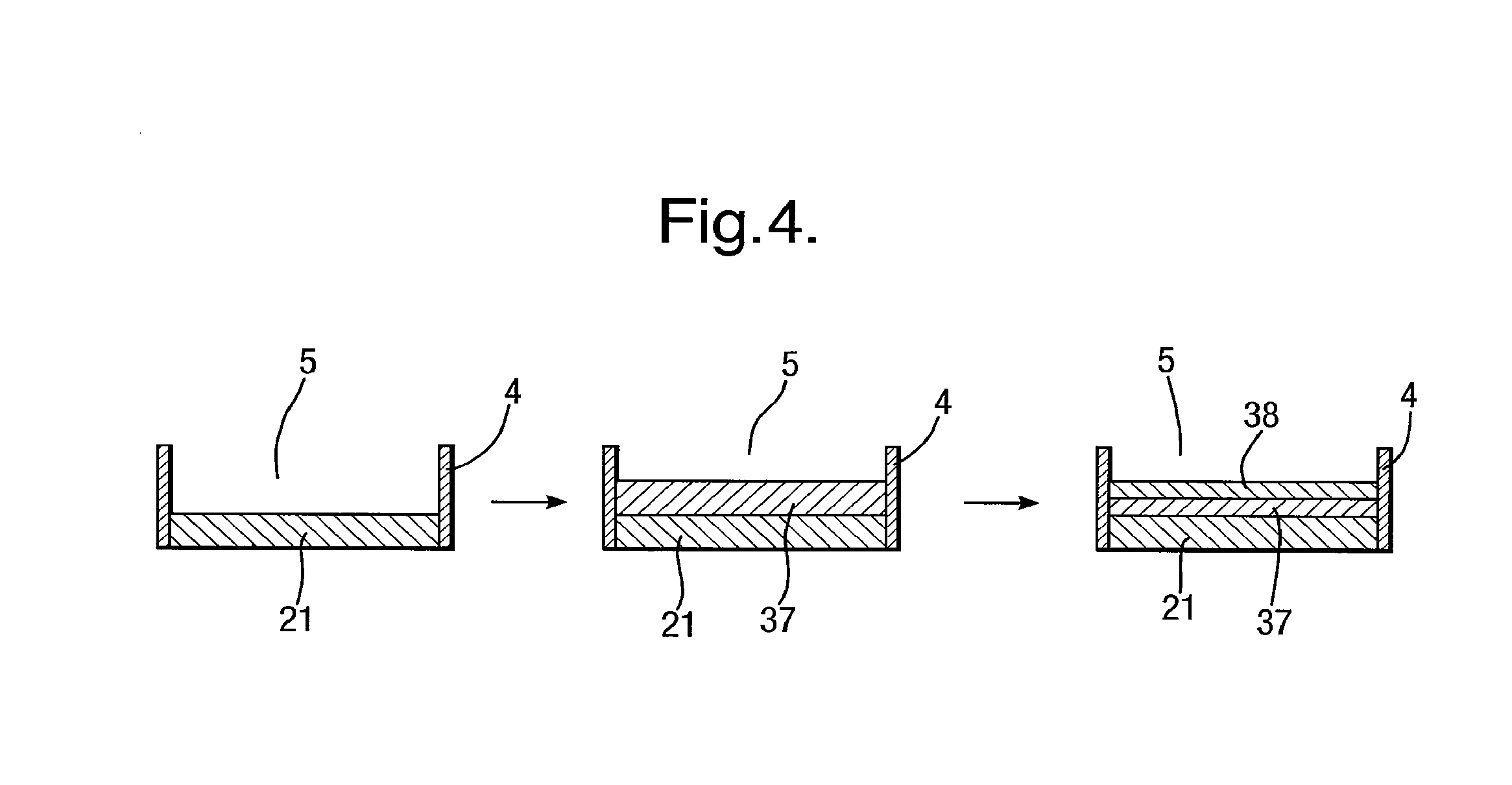
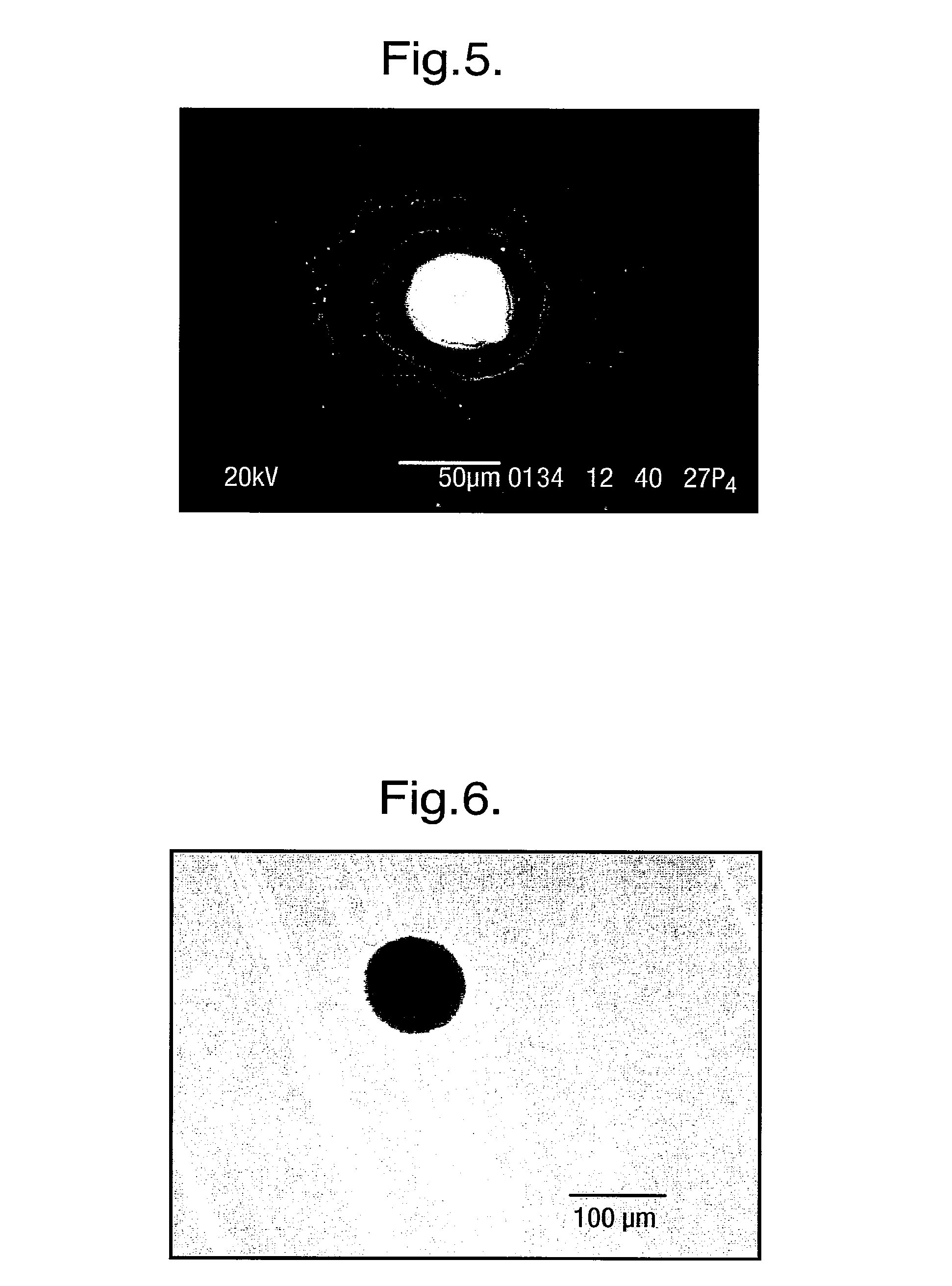
[0219]An example will now be described for a typical apparatus 1, in which the first conductive layer 20 was formed by a silver foil strips (25 μm thick, from Goodfellow) thermally laminated onto the substrate 3 using a 15 μm thick laminating film (Magicard) to form the further layer 4. A circular recess 5 of diameter 100 μm was created further layer 4 using an excimer laser, exposing a circular silver electrode 21 of diameter 100 μm. The exposed silver was chloridised electrochemically as described previously. The second conductive layer 23 was a screen printing silver / silver chloride ink printed on the top side of the body 2.
[0220]The pre-treatment coating 30, comprising 0.5 μl of 1% heaxadecane +0.6 mg / ml DPhPC in pentane, was then applied to the body 2 and dried at room temperature.
[0221]The cover 6 comprised a 1 mm thick silicon rubber body with a 250 μm thick Mylar lid. Lipid (4 μl of 10 mg / ml DPhPC in pentane) was applied to the inside of the cover 6 and allowed to dry at roo...
example 3
[0236]There will now be discussed modifications to the apparatus 1 to include plural recesses 5, commonly referred to as an array of recesses 5. The ability to easily form an array of layers 11 across an array of recesses 5 in a single apparatus 1 is a particular advantage of the present disclosure. By contrast to traditional methods of formation of lipid bilayers, the apparatus 1 has a single chamber 7, but creates the layer 11 in situ during the test and captures a reservoir of electrolyte in the recess 5 under the layer 11 which allows continuous stable measurement of current passing through protein pores inserted in the layer 11. Further the layer 11 formed is of high quality and is localised to the area of the recess 5, ideal for high-fidelity current measurements using membrane protein pores. These advantages are magnified in an apparatus 1 which forms an array of layers 11 because this allows measurements to be taken across all the layers 11 in parallel, either combining the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| resistance | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com