Method for generating oxygen and water electrolysis device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
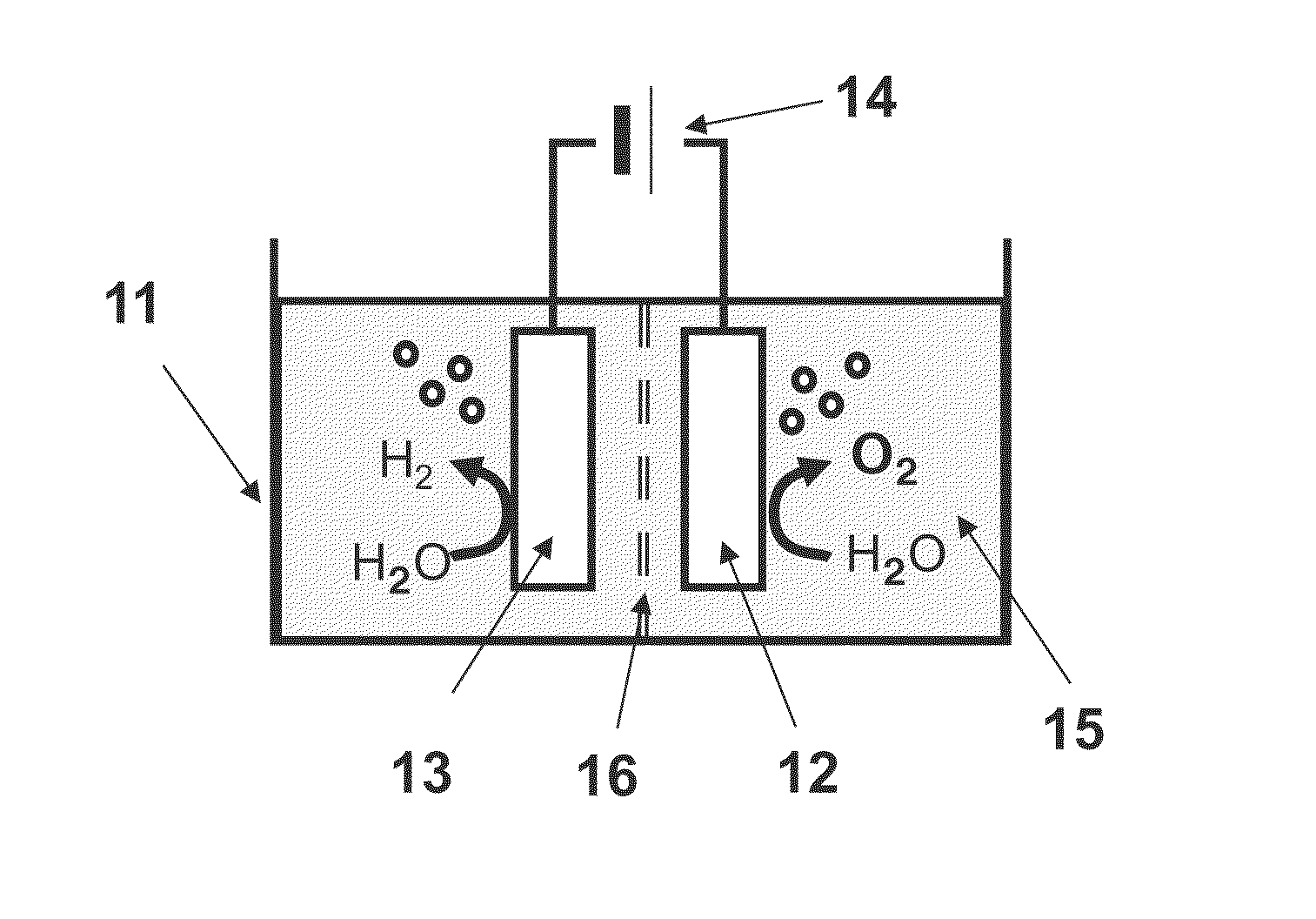
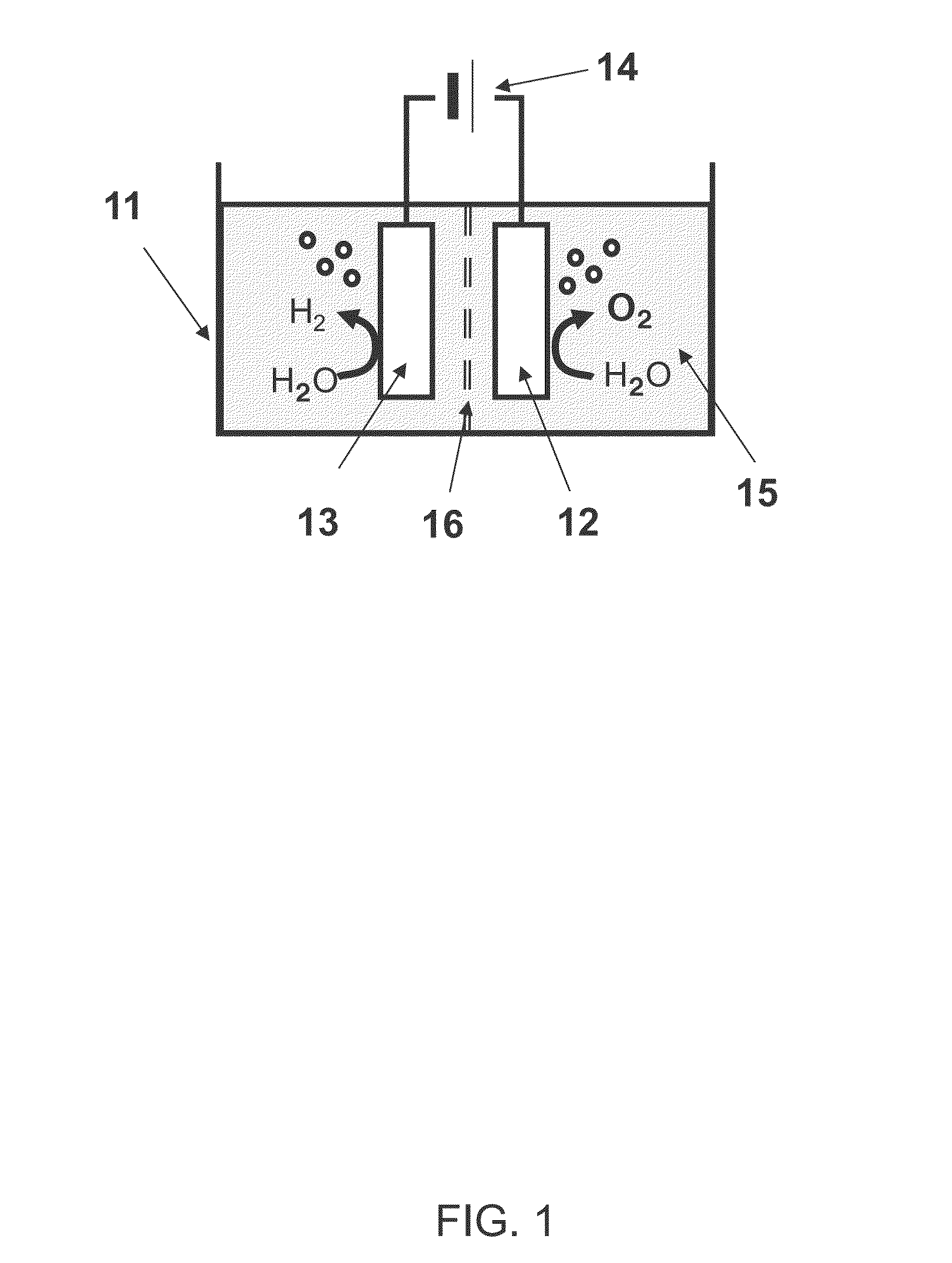
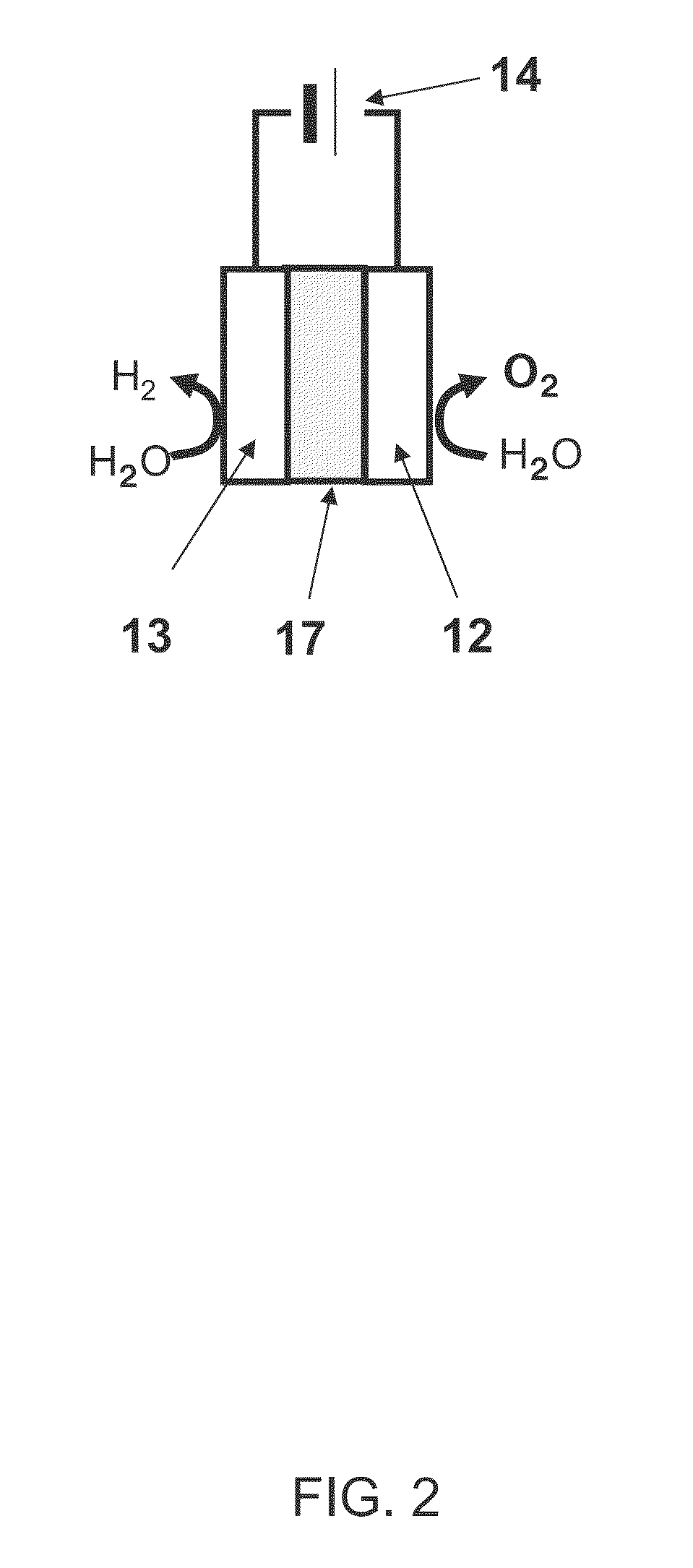
[0029]FIG. 1 shows a schematic view of a water electrolysis device 100 according to a first embodiment. The water electrolysis device 100 according to the first embodiment comprises a container 11, an anode 12, a cathode 13, and a power supply 14.
[0030](Container 11)
[0031]An electrolyte aqueous solution 15 is stored in the container 11. An example of the electrolyte aqueous solution 15 is an alkaline aqueous solution such as potassium hydroxide or sodium hydroxide. Water is electrolyzed using the alkaline aqueous solution to improve the efficiency of the oxygen generation and to decrease an electric power necessary for the electrolysis.
[0032]Another example of the electrolyte included in the electrolyte aqueous solution 15 is sulfuric acid, nitric acid, or perchloric acid. More specifically, an example of a cation of the electrolyte included in the electrolyte aqueous solution 15 is a proton, an alkali metal ion, or an alkaline earth metal ion. An example of an anion of the electrol...
example 1
Preparation of the Anode 12
[0053]The anode 12 according to the example 1 was made by supporting a copper cobalt delafossite compound on a conductive carbon substrate.
[0054]First, the copper cobalt delafossite compound was prepared by a hydrothermal synthesis method.
[0055]Particularly, cobalt hydroxide represented by the chemical formula Co(OH)2 (available from Wako Pure Chemical Industries, Ltd.) was headed under an oxygen atmosphere at a temperature of 120 Celsius degrees for twenty-four hours to give cobalt oxyhydroxide represented by the chemical formula CoOOH. Cobalt oxyhydroxide represented by the chemical formula CoOOH (0.25 grams) and copper(I) oxide represented by the chemical formula Cu2O (available from Wako Pure Chemical Industries, Ltd., 0.39 grams) were mixed with a sodium hydroxide aqueous solution (40 milliliters) having a concentration of 2 mol / liter to give a mixture. The mixture was poured in a Teflon vessel having a volume of 100 milliliters. Then, the mixture was...
reference example 1
[0092]An anode supporting cobalt oxide represented by the chemical formula Co3O4 was prepared as below and the overvoltage thereof was calculated.
[0093]Cobalt oxide represented by the chemical formula Co3O4 (available from Furuuchi Chemical Corporation, 40 milligrams) was dispersed in pure water (2 milliliters) to prepare a slurry. Similarly to the case of the example 1, an anode was prepared using the slurry and the oxygen generation property thereof was evaluated. The curve (g) in FIG. 4 is an electric current—voltage property of the anode according to the reference example 1. The anode according to the reference example 1 had an electric potential difference EPD1 of 1.60 volts. Therefore, the anode according to the reference example 1 had an overvoltage of 0.37 volts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com