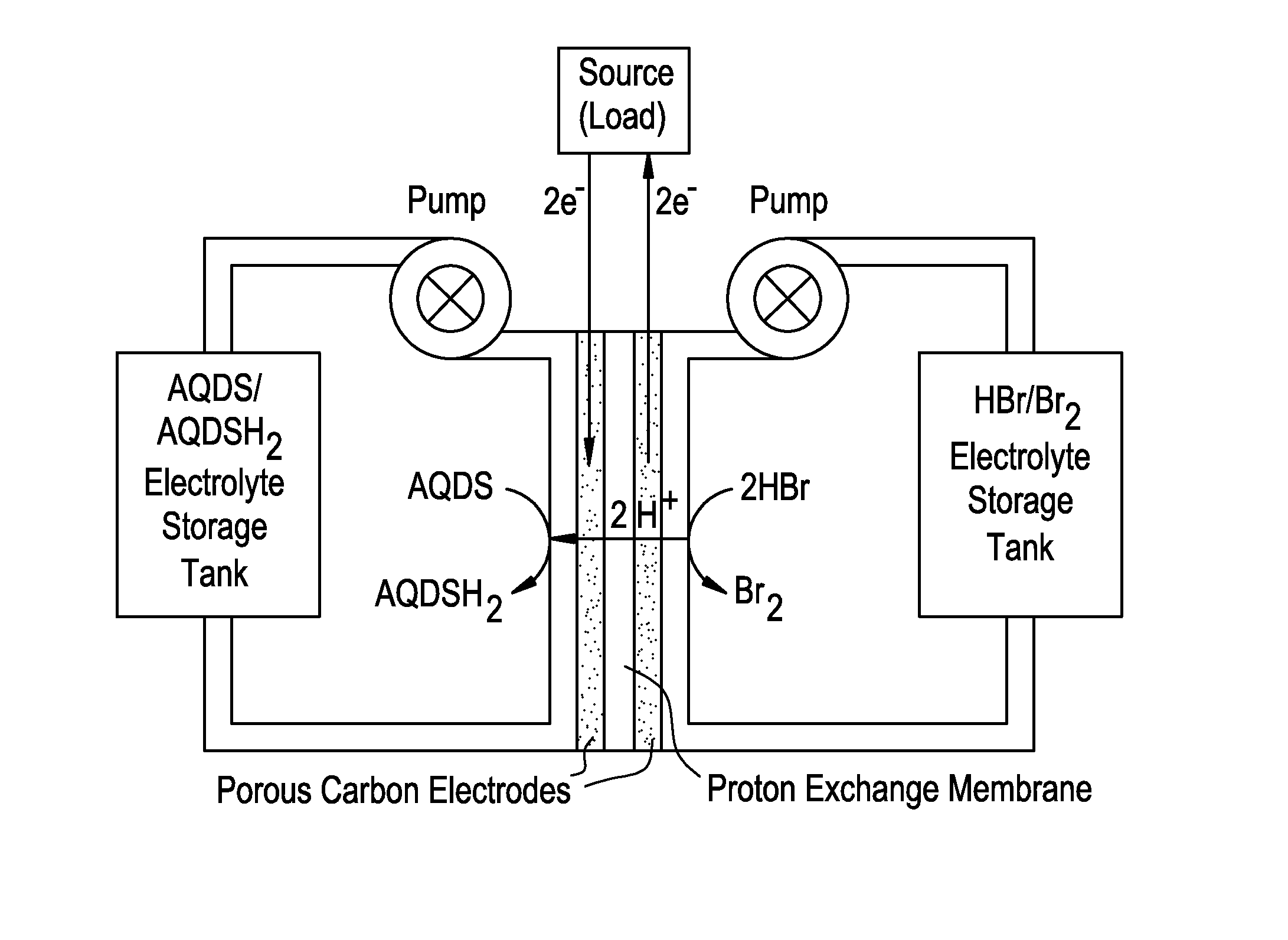
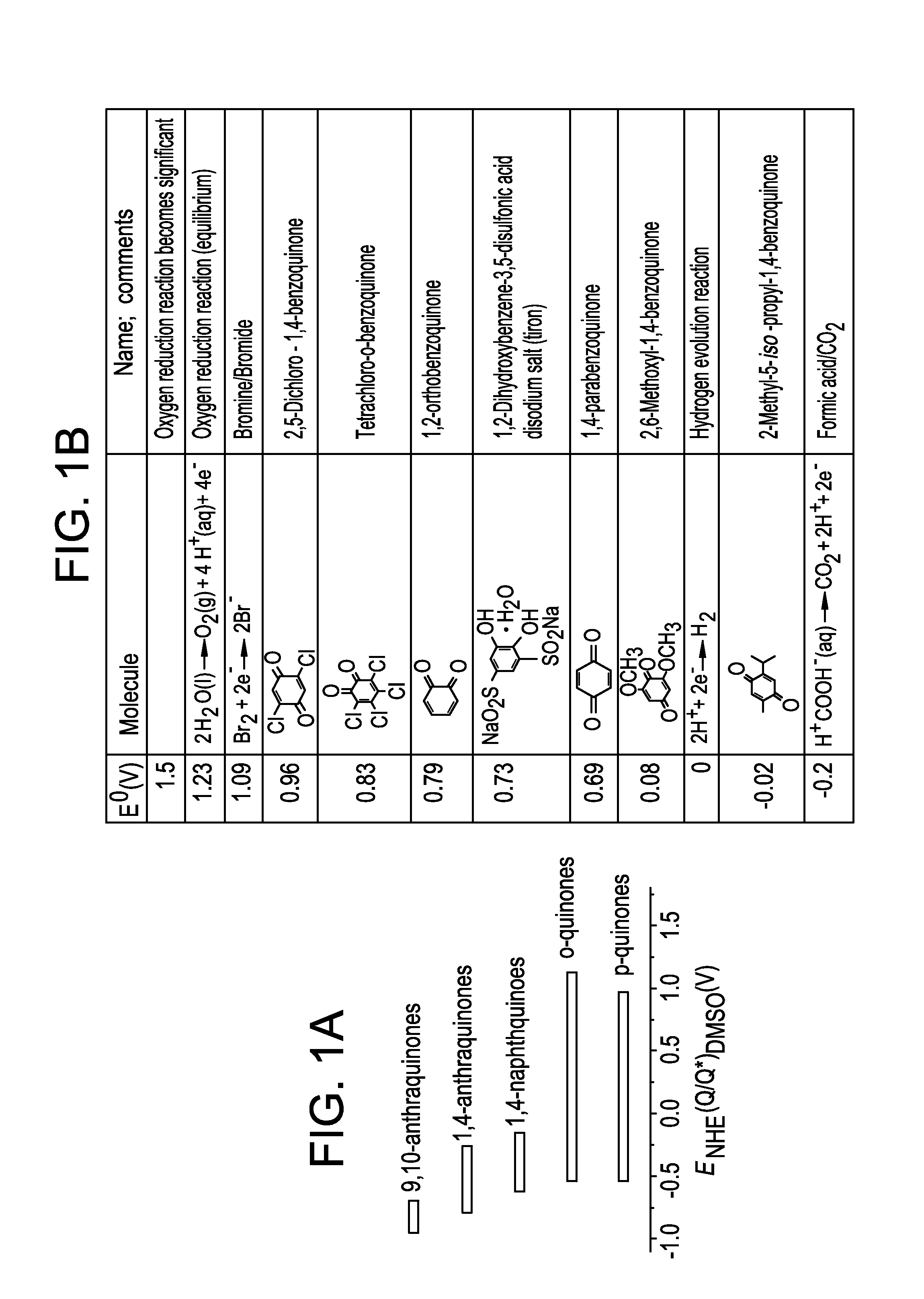
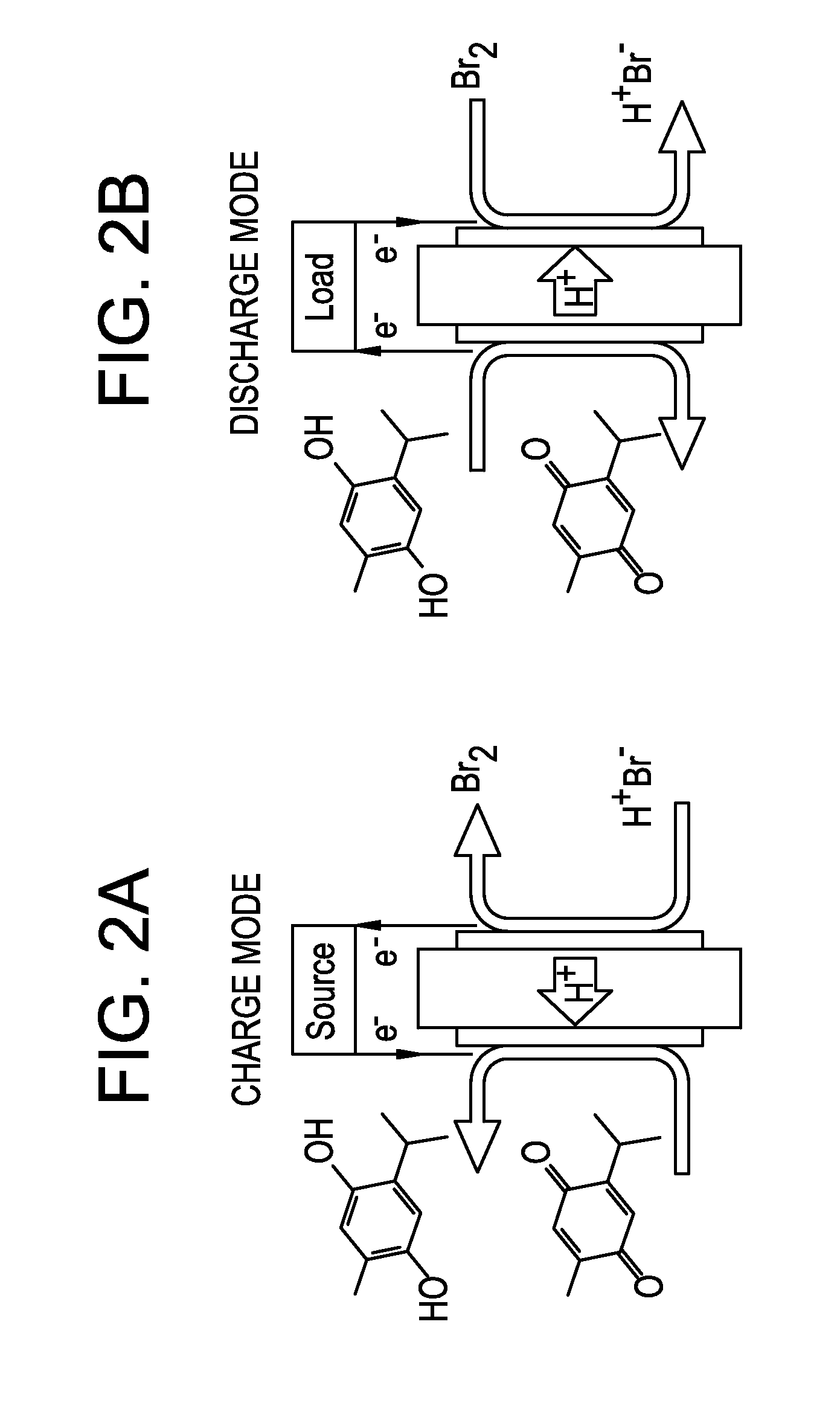
Small organic molecule based flow battery
a flow battery and organic molecule technology, applied in the direction of indirect fuel cells, non-aqueous electrolyte cells, cell components, etc., can solve the problems of compromising the effectiveness of the electrode, limiting the use in a manned environment, such as the home, and limiting the price at large scale. , to achieve the effect of high current density, high efficiency and large-scale energy storag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0077]1 molal 1,2-ortho-benzohydroquinone (catechol) was oxidized in 1 N H2SO4 at a flat Pt electrode, obtaining the cyclic voltammetry curves shown in FIG. 3a. The sweep starts at (0.2 V, 0 mA / cm2) and proceeds at 25 mV / s to the right. At about 600 mV vs. Ag / AgCl (the known E0 is 795 mV vs. SHE), the current density increases as catechol is oxidized to the orthoquinone form. The oxidative current density peaks at about 150 mA / cm2. The peak and downturn are caused by reactant depletion in a quiescent (non-flowing, non-stirred) electrolyte. In a test at a higher concentration of 3.9 molal (FIG. 3b), we observe asymmetric oxidation and reduction peaks, achieving current densities above 500 mA / cm2 for the former. The asymmetric shape of the curve in FIG. 3b arises because the quinone form is unstable in aqueous solution. In addition the limited solubility of ortho-benzoquinone (0.06 M) compared to its reduced form precludes symmetric behavior at high concentration.
example 2
[0078]The half-cell redox behavior of hydroquinone-2-sulfonic acid (HQSA) is shown in FIG. 4. At a pH of 7 a rise in current density was observed beginning near 0.5 V and peaking at higher voltage. Upon reversing the direction of the voltage sweep, negative current (indicating a reduction event) was observed near 0.3 V. The large difference between where the oxidation and reduction currents are observed indicates a chemical process was likely occurring. In this case, upon oxidation of HQSA to the quinone form, water reacted with the quinone to form a new species. This species was reduced at the lower 0.3 V potential. At a pH of 13, the reaction became rapid and reversible because in basic solution the —OH groups on HQSA became deprotonated. The positive and negative current density observed near 0 V was indicative of a 2-electron redox event with no protons having been exchanged.
example 3
[0079]AQDS was subjected to half-cell electrochemical measurements. Cyclic voltammetry of a 1 mM solution of AQDS in 1 M sulfuric acid on a glassy carbon disc working electrode showed current peaks corresponding to reduction and oxidation of the anthraquinone species (FIG. 5a).
[0080]The peak separation of 34 mV was close to the 59 mV / n, where n was the number of electrons involved, expected for a two-electron process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com