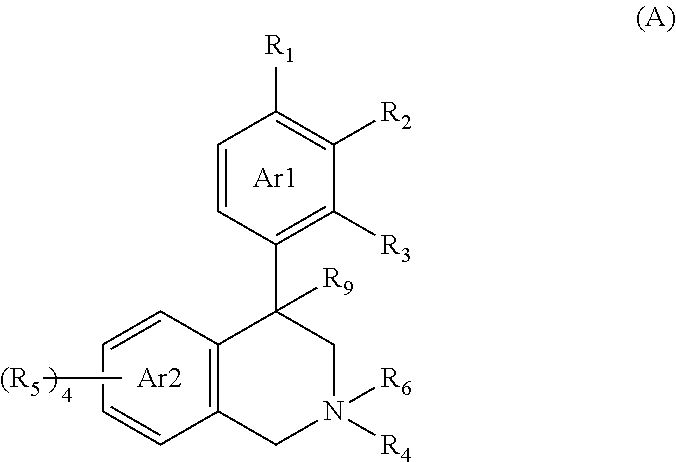
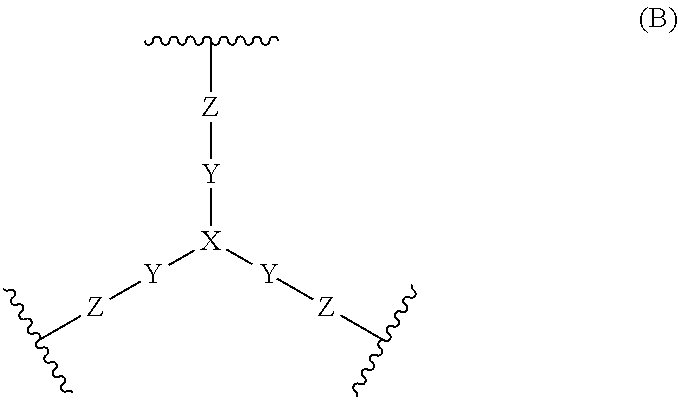
Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders
a technology of gastrointestinal tract disorders and compounds, which is applied in the direction of drug compositions, peptides/protein ingredients, extracellular fluid disorders, etc., can solve the problems of fluid transudation into the lungs and congestive symptoms, increased filling pressure, and shortness of breath
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N1,N7-bis(2-(2-(2-(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)ethoxy)ethoxy)ethyl)-4-(3-(2-(2-(2-(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)ethoxy)ethoxy)ethylamino)-3-oxopropyl)-4-nitroheptanediamide
[0360]
[0361]Example 1: To a solution of A (972 mg, 193 mmol) and DIEA (657 μL, 3.87 mmol) in DCM (20 mL) was added bis(perfluorophenyl) 4-nitro-4-(3-oxo-3-(perfluorophenoxy)propyl)heptanedioate (intermediate B, 500 mg, 0.645 mmol) and the resulting solution stirred at room temperature for 20 h. The solvent was removed under reduced pressure and the resulting residue purified by automated flash column silica gel chromatography using a gradient of DCM:MeOH (99:1 to 9:1) to give the title compound as a yellow solid (516 mg, 46%) after the solvent was removed. 1H-NMR (400 MHz, CD3OD) δ 7.79-7.75 (m, 3H), 7.70 (t, J=1.5 Hz, 3H), 7.53 (t, J=7.6 Hz, 3H), 7.50-7.45 (m, 3H), 7.34 (d, J=2.1 Hz, 3H), 6.80 (s, 3H), 4.44-...
example 2
4-amino-N1,N7-bis(2-(2-(2-(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)ethoxy)ethoxy)ethyl)-4-(3-(2-(2-(2-(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)ethoxy)ethoxy)ethylamino)-3-oxopropyl)heptanediamide
[0362]
[0363]Example 2, method A: To a solution of N1,N1-bis(2-aminoethyl)ethane-1,2-diamine (1.21 mL, 8.06 mmol) in DMF (2 mL) was slowly added a solution of intermediate D (7.75 g, 4.03 mmol) in DMF (10 mL) and the resulting mixture stirred for 30 min at room temperature. The solution was cooled to 0° C. and 1 M aqueous TFA was added (20 mL), until the solution reached pH=1. The solution was then diluted with 1:1 MeCN:H2O to give a final volume of 60 mL. The solution was purified by automated flash column reverse phase chromatography using a gradient of H2O 0.05% TFA: CH3CN 0.05% TFA (80:20 to 60:40) and detection by UV at 254 nm in three batches. The fractions containing pure material were concentrated and ...
example 3
1-(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)-13,13-bis(3-(2-(2-(2-(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)ethoxy)ethoxy)ethylamino)-3-oxopropyl)-10-oxo-3,6-dioxa-9,14-diazahexadecane-16-sulfonic acid
[0365]
[0366]Example 3: Taurine (9.2 mg, 0.074 mmol) was dissolved in H2O (200 μL), to which was added DIEA (26 μL, 0.15 mmol), followed by DMF (800 μL). To the resulting solution was added N,N′-disuccinimidyl carbonate (19 mg, 0.074 mmol) and the solution stirred at 50° C. for 1. Example 2 (25 mg, 0.015 mmol) was then added and the solution stirred for 18 h at 50° C. The solution was then diluted with H2O and acidified with TFA, then purified by preparative HPLC with a C18 silica gel stationary phase using a gradient of H2O 0.05% TFA:CH3CN 0.05% TFA (80:20 to 40:60) and detection by UV at 254 nm to give the title compound tri-TFA salt (10 mg, 30% yield) as a white solid. 1H-NMR (400 MHz, CD3OD) δ 7.91-7.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com