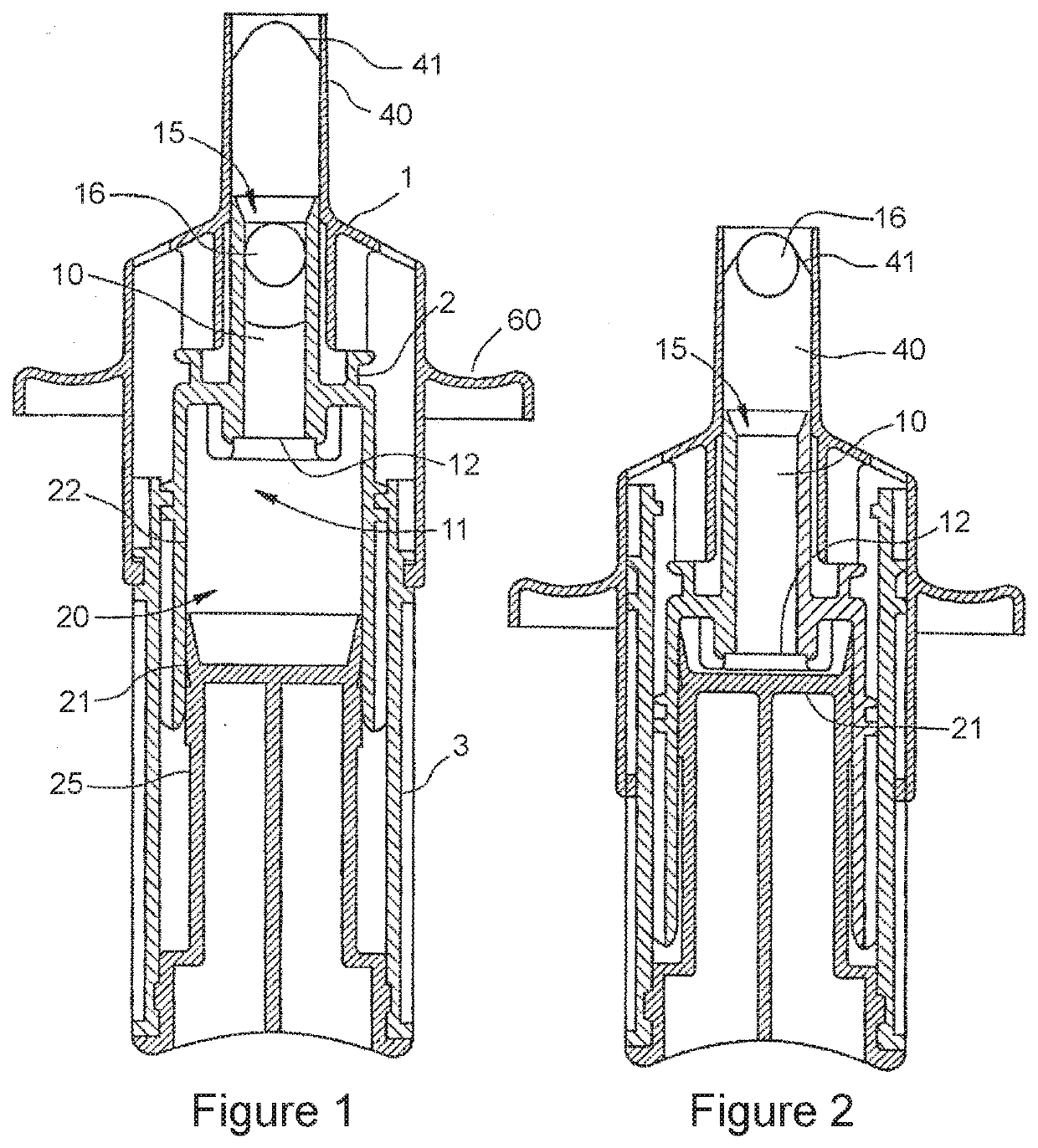
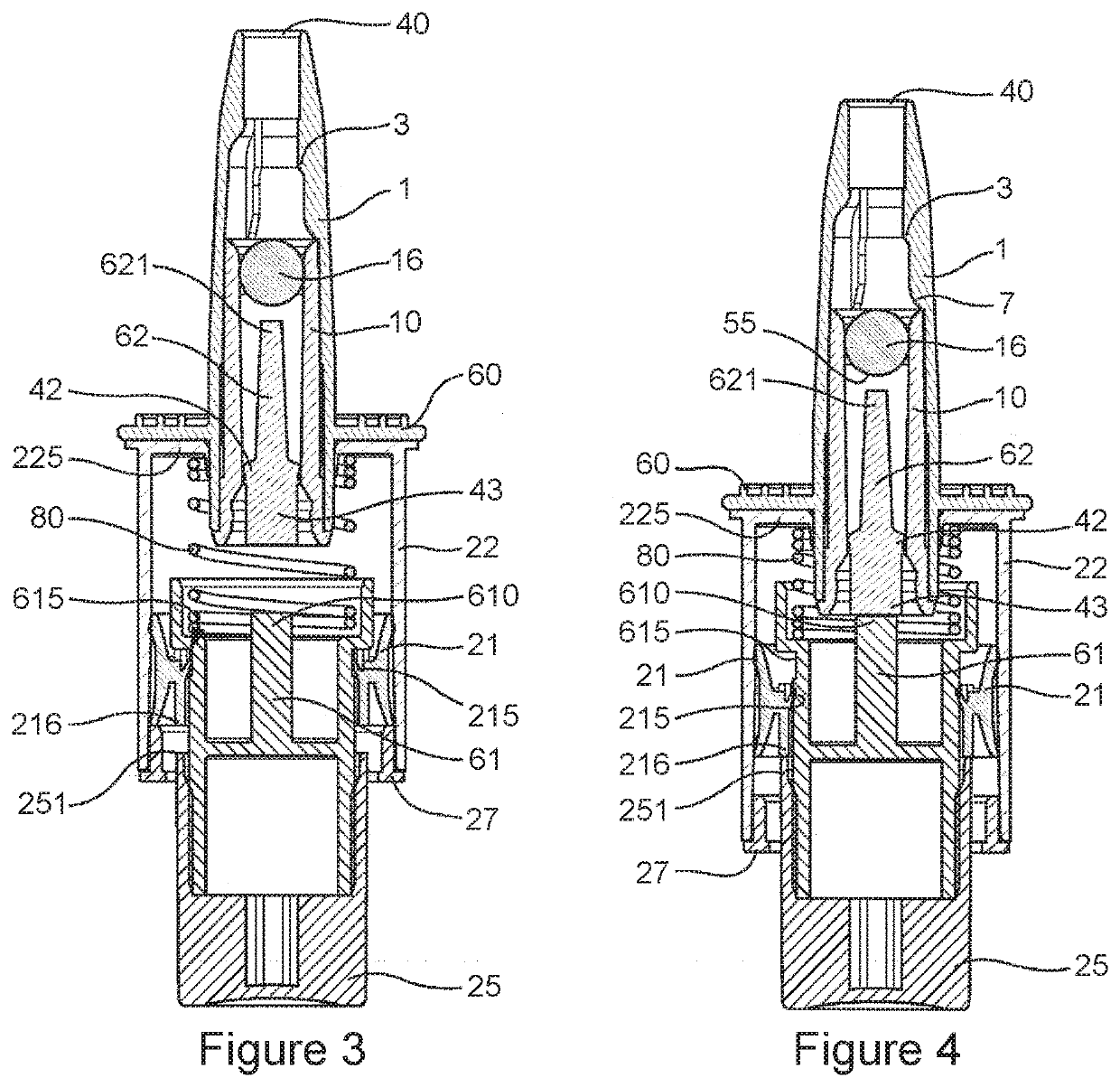
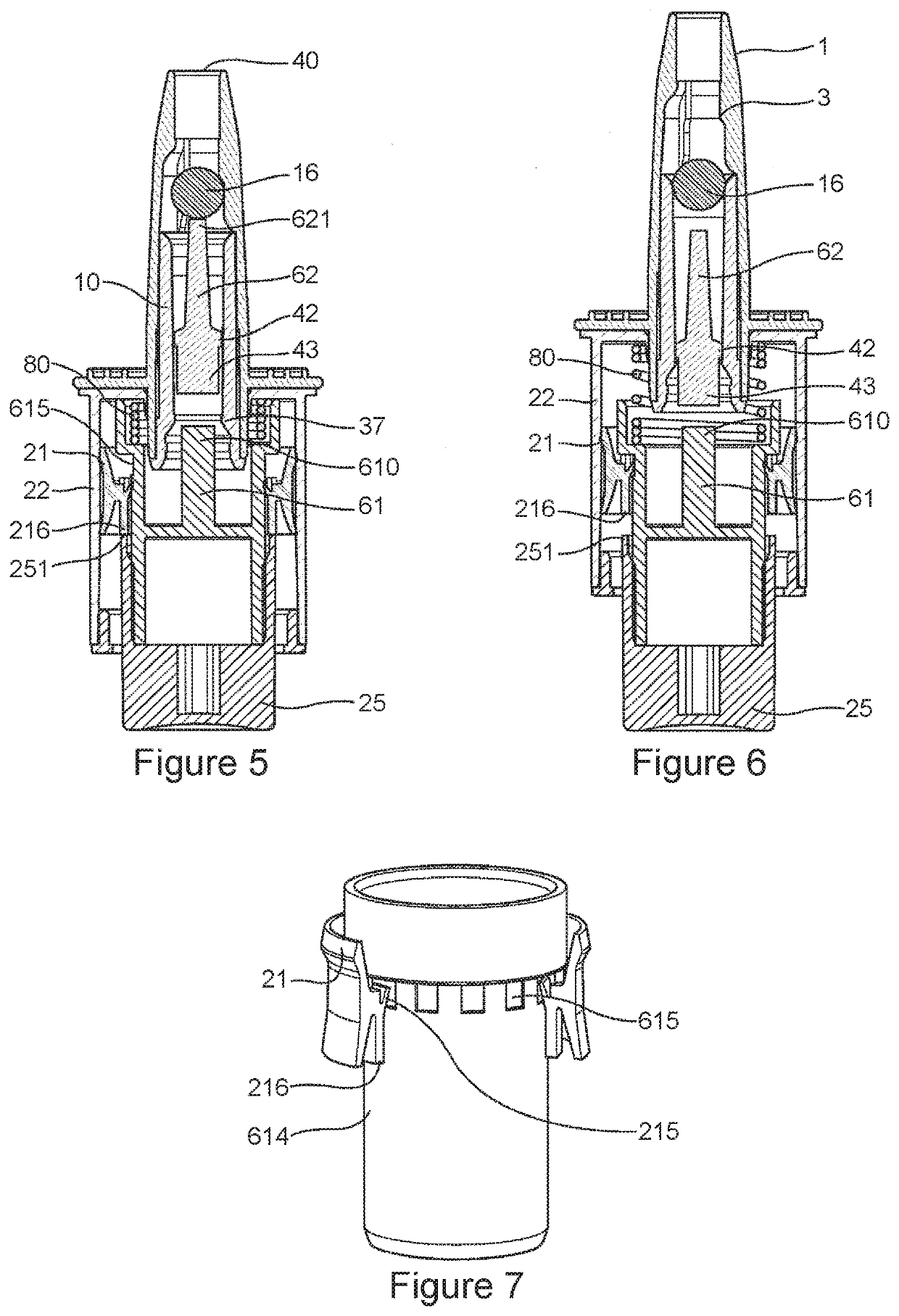
Pharmaceutical composition for nasal delivery
a technology of pharmaceutical composition and nasal delivery, which is applied in the direction of granular delivery, medical preparations, organic active ingredients, etc., can solve the problems of drug addiction worldwide, high risk, and high addiction of opioids and opiates, and achieves higher bioavailability of opioid antagonists, physical and chemical stability, and high bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Physical Stability of Spray-Dried Powders
[0231]In order to assess the physical stability and mitigate the risk for crystallization during storage, glass transition temperatures (Tg) were determined using differential scanning calorimetry (DSC) and are presented in Table 3 below.
[0232]Compositions were prepared generally in accordance with the procedure described in Example 1 above, using mannitol, trehalose and lactose as carrier materials.
[0233]For lactose, the true Tg, as well as that of the formulation subjected to equilibration at four different RH conditions at 25° C. (10%, 20%, 30% and 40% RH), were measured (although 30% was not logged). For mannitol and trehalose, Tg was measured as received (‘Ambient’) and after drying (‘Dried’).
[0234]For the dried samples, the DSC ampoule lid was punched automatically immediately before start of the DSC run, introducing a hole of about 0.3 mm diameter. The purpose of this was to allow any remaining moisture to evaporate before the glass tr...
example 3
Ex-vivo Evaluation of Nasal Mucosal Absorption of Naloxone and Nalmefene
[0236]A standard static diffusion (Franz) cell set up was employed to set up an ex vivo model for nasal mucosa absorption, using an excised porcine nasal tissue.
[0237]Solutions containing naloxone HCl dihydrate, nalmefene HCl, benzalkonium chloride (Sigma-Aldrich Sweden AB), sucrose monolaurate (IMCD Nordic AB) and / or polysorbate 80 (Croda Nordica AB) were prepared by standard techniques, to provide formulations according to Table 4 below. Potassium phosphate buffer (Sigma-Aldrich Sweden AB) was added to give the pH stated in Table 4 below.
TABLE 4Formulation12345678naloxone (mg / mL)5555555nalmefene (mg / mL)5555555benzalkonium chloride (mg / mL)0.10.10.1sucrose monolaurate (mg / mL)0.42polysorbate 80 (mg / mL)2pH4.54.54.54.54.54.54.56.5
Diffusion through the tissue was measured after 7 hours and permeation is shown as mean (three repeats) cumulative transport (μg / cm2; with SD) in FIGS. 8 and 9 for naloxone and nalmefene, ...
example 4
Naloxone-Containing Composition B
[0239]The general procedure described in Example 1 was employed to make a spray-dried composition from naloxone HCl dihydrate (1.199 g), along with α-D-lactose monohydrate (5.026 g; DFE Pharma Germany), sucrose monolaurate D-1216 (0.062 g; Mitsubishi-Kagaku Foods Corporation, Japan).
[0240]Composition B comprised a single dose of naloxone of 4 mg (calculated as the HCl salt).
[0241]Devices were placed in heat-sealed aluminum pouches (Protective Packaging, UK) before use.
[0242]The geometric particle size distribution (PSD) was measured using a Malvern Mastersizer 2000 (Malvern Panalytical Ltd, UK) and aerodynamic particle size distribution (aPSD) using a fast screening impactor (FSI, Copley Scientific, UK). PSD: d10=15 μm and d90=55 μm; aPSD <5 μm=0%.
[0243]General method for PSD measurements: 80 to 100 mg of sample was dispersed in 5 mL of silicone oil and mixed well before sonication for 20 to 30 seconds. Three measurements were made on the solution us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com