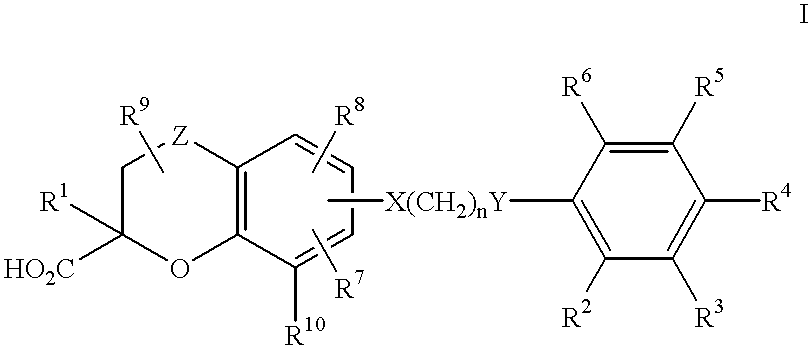
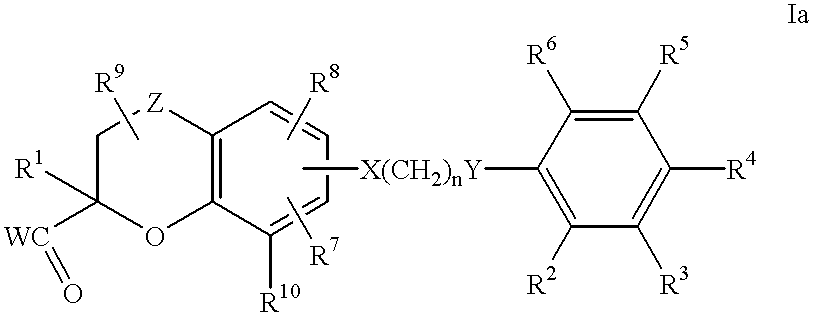
Benzopyrancarboxylic acid derivatives for the treatment of diabetes and lipid disorders
a technology of benzopyrancarboxylic acid and derivatives, which is applied in the field ofbenzopyrancarboxylic acid derivatives for the treatment of diabetes and lipid disorders, can solve the problems of increased and premature morbidity and mortality, increased risk of macrovascular and microvascular complications in patients with type 2 diabetes mellitus, and increased risk of macrovascular and microvascular complications, etc., to achieve the effect of effective treatment and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
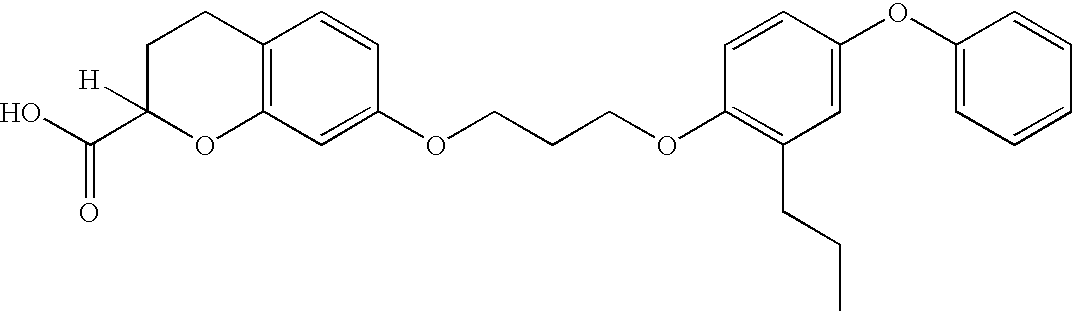
7-(3-(2-Propyl-4-phenoxyphenoxy)propoxy)-chromane-2-carboxylic acid
##STR33##
Step A: Ethyl-7-hydroxychromane-2-carboxylate ##STR34##
To a large hydrogenation vessel were added ethyl 7-hydroxychromone-2-carboxylate (=ethyl 7-hydroxy-4-oxo-4H-chromene-2-carboxylate) (675.4 g, 2.88 mol), EtOH 4 liters, conc. hydrochloric acid 40 ml. The resulting suspension was combined with 5% Pd / C 68 g, and subjected to hydrogenation condition (H.sub.2, 40 psi, rt) overnight. The reaction mixture was filtered through a pad of celite to remove the catalyst. The filtrate was concentrated to give thick oily material, which solidified upon standing. Tan solid 630.1 g (98%).
.sup.1 H-NMR (500 MHz, CDCl.sub.3): .delta. 6.89 (d, 1H, J=8.2 Hz), 6.46 (d, 1H, J=2.5 Hz), 6.4 (dd, 1H, J=2.5, 8.2 Hz), 4.9 (brs, 1H), 4.71 (dd, 1H, J=3.1, 7.5 Hz), 4.27 (q, 2H, J=7.3 Hz), 2.76 (m, 1H), 2.7 (m; 1H), 2.25 (m, 1H), 2.18 (m, 1H), 1.3 (t, 3H, J=7.2 Hz).
Step B: Ethyl 7-(3-(2-propyl-4-phenoxyphenoxy)propoxy)-chromane-2-carbox...
example 2
7-(3-(2-Propyl-4-phenoxyphenoxy)propoxy)-2-propylchromane-2-carboxylic acid
##STR35##
Step A: Ethyl 7-(3-(2-propyl-4-phenoxyphenoxy)propoxy)-2-propylchromane-2-carboxylate
To a 2 ml THF solution of ethyl 7-(3-(2-propyl-4-phenoxyphenoxy)propoxy)-chromane-2-carboxylate (Example 1, Step B) (100 mg, 0.204 mmol) were added hexamethylphosphoramide (0.046 ml, 0.26 mmol) and sodium bis(trimethylsilyl)amide (1.0M THF solution) (0.265 ml, 0.265 mmol) upon cooling in a dry ice-acetone bath. After stirring at that temperature for 30min, to it was added iodopropane (0.06 ml, 6.2 mmol). The cooling bath was removed and the reaction mixture was gradually warmed to rt overnight. The solvent was removed under reduced pressure, diluted with AcOEt and water. The organic layer was separated. The aqueous layer was extracted twice with AcOEt. The combined organic layers were dried over anhydrous Na.sub.2 SO.sub.4, filtered, concentrated, and chromatographed on silica gel. Elution with 5% AcOEt / hexanes gave ...
example 3
7-(4-(2-Propyl-4-phenoxyphenoxy)butoxy)-chromane-2-carboxylic acid
##STR36##
The title compound was prepared following the procedure in Example 1, Steps A-C using 4-(4-bromobutoxy)-3-propylphenyl phenyl ether (U.S. Pat. No. 6,008,237) instead of 4-(3-bromopropoxy)-3-propylphenyl phenyl ether.
.sup.1 H-NMR (500 MHz, CDCl.sub.3): .delta. 7.31 (m, 2H), 7.04 (t, 1H, J=7.4 Hz), 6.96 (m, 3H), 6.88 (d, 1H, J=2.5 Hz), 6.84-6.79 (m, 2H), 6.53 (m, 2H), 4.73 (dd, 1H, J=3.2, 8.7 Hz), 4.03 (m, 4H), 2.85 (m, 1H), 2.75 (m, 1H), 2.59 (m, 2H), 2.34 (m, 1H), 2.15 (m, 1H), 2.0 (m, 1H), 1.6 (sext, 2H, J=7.5 Hz), 0.94 (t, 3H, J=7.4 Hz). ms: m / e=477 (M+1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com