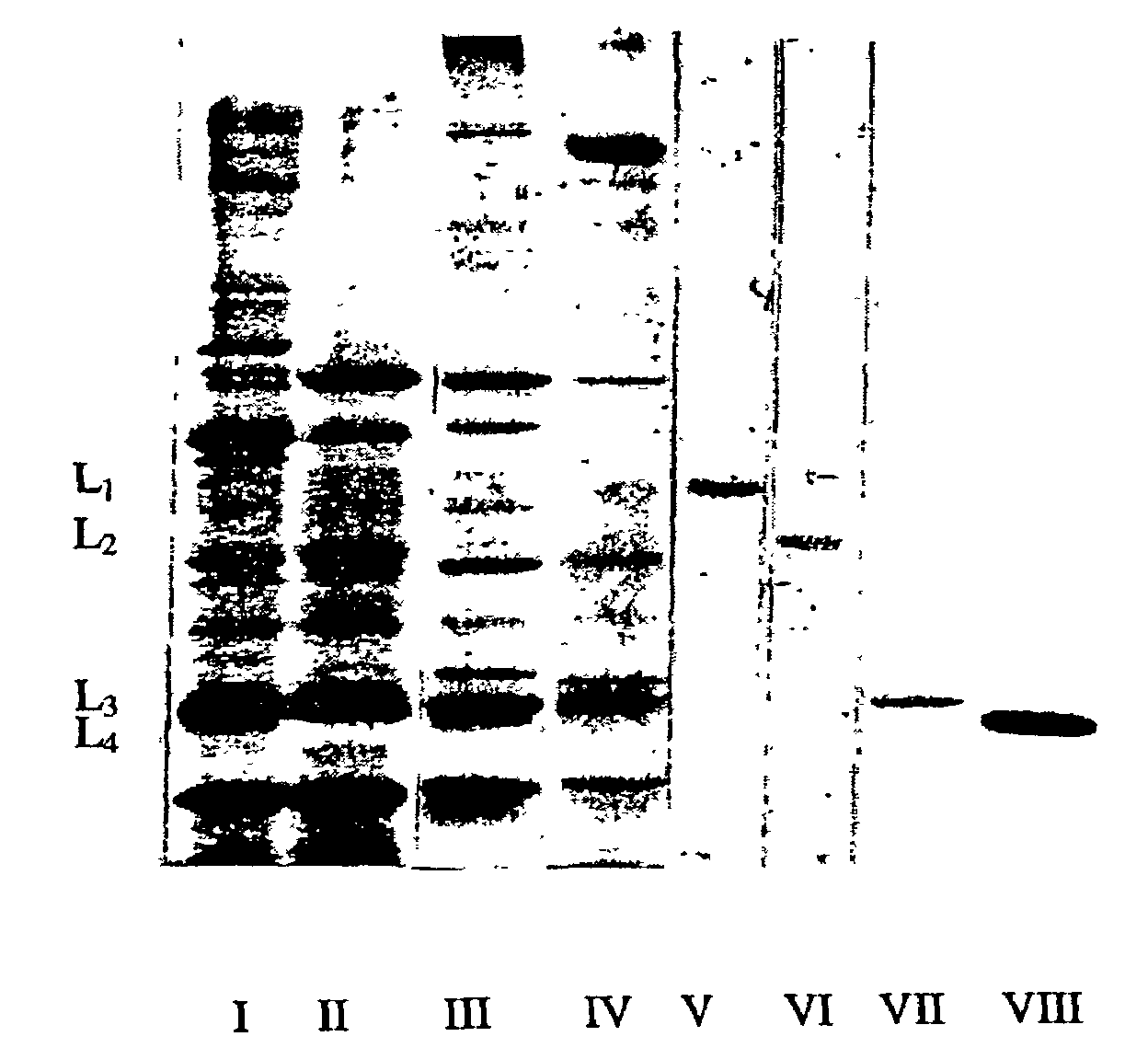
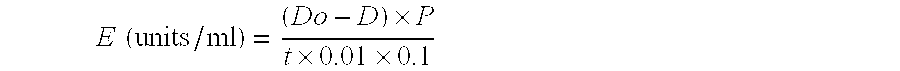Bacteriolytic complex, method for producing said complex and strain for carrying out said method
a bacteriolytic complex and complex technology, applied in the field of bacteriolytic complexes, can solve the problems of insufficient bacteriolytic activity, unstable application in laboratory practice, insufficient stability of lyzozyme, etc., and achieve the effect of increasing the yield of the target product and reducing the duration of the end product obtaining process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0102]The strain Lysobacter sp. XL1 is cultivated as described in Example 1 on a medium of the following composition, g / l:
[0103]
Glucose 5.0Peptone 2.0Yeast autolysate100 mlNa2HPO4 × 12H2O 4.2KH2PO4 1.0KCl 0.6MgSO4 × 7H2O 5.0FeSO4 × 7H2O 0.1Waterup to 1 liter.
[0104]By hour 24 of cultivation, the bacteriolytic activity of culture liquid is 70 LU / ml.
example 3
[0105]The strain-producer is cultivated as described in Example 1 on a medium of the following composition, g / l:
[0106]
Glucose5.0Peptone2.0Yeast extract2.0Na2HPO4 × 12H2O4.2KH2PO41.0KCl0.6MgSO4 × 7H2O5.0FeSO4 × 7H2O0.1Waterup to 1 liter.
[0107]By hour 24 of cultivation, the bacteriolytic activity of culture liquid is 90 LU / ml.
example 4
[0108]Ankum fermenter is filled with 6 liters of a medium of the following composition (g / l):
[0109]
Glucose5.0Peptone2.0Yeast extract2.0Na2HPO4 × 12H2O4.2KH2PO41.0KCl0.6MgSO4 × 7H2O5.0FeSO4 × 7H2O0.1Waterup to 1 liter.Sterilized at 0.5 kg-f / cm2 for 30 min.
[0110]300 ml of inoculum grown on the above medium is introduced into the fermenter under sterile conditions. Cultivation is run at 29° C., air feeding of 0.3 v per 1 v of the medium per 1 min, so that the partial pressure of oxygen in the medium would not fall below 30% of saturation.
[0111]The pH of the medium is maintained in the range of 6.3–7.5, with 10% NaOH solution or 10% HCl solution fed to the medium when pH decreases or increases, respectively. By hour 24 of cultivation, the activity of culture liquid is 70 LU / ml.
[0112]Cooled culture liquid (5.6 1) is supplemented with ammonium sulfate to 80% saturation, left over for 12 h, and precipitate is separated by centrifugation. The precipitate is dissolved in water, dialyzed, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com