Surfactant and cleaning compositions comprising microbially produced branched fatty alcohols
a technology of branched fatty alcohols and surfactants, which is applied in the direction of ani cati non-ionic surface active compounds, etc., can solve the problems of short shelf life and corrosion of long-chain alkyl surfactants, and inability to meet the requirements of cleaning compositions, etc., to achieve the branched material of crude petroleum requires a significant financial investmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Constructing E. coli MG1655 ΔfadE_ΔtonA AAR:kan
[0473]This example describes the construction of a genetically engineered microorganism in which the expression of a fatty acid degradation enzyme is attenuated.
[0474]The fadE gene of E. coli MG1655 was deleted using the lambda red system described by Datsenko et al., Proc. Natl. Acad. Sci. USA 97: 6640-6645 (2000), with the following modifications:
[0475]The following two primers were used to create the deletion of fadE:
[0476]
Del-fadE-F(SEQ ID NO: 158)5′-AAAAACAGCAACAATGTGAGCTTTGTTGTAATTATATTGTAAACATATTGATTCCGGGGATCCGTCGACC;andDel-fadE-R(SEQ ID NO: 159)5′-AAACGGAGCCTTTCGGCTCCGTTATTCATTTACGCGGCTTCAACTTTCCTGTAGGCTGGAGCTGCTTC
[0477]The Del-fadE-F and Del-fadE-R primers were used to amplify the kanamycin resistance (KmR) cassette from plasmid pKD13 by PCR. The PCR product was then used to transform electrocompetent E. coli MG1655 cells containing pKD46 that had been previously induced with arabinose for 3-4 hours. Following a 3-hour outgrowt...
example 2
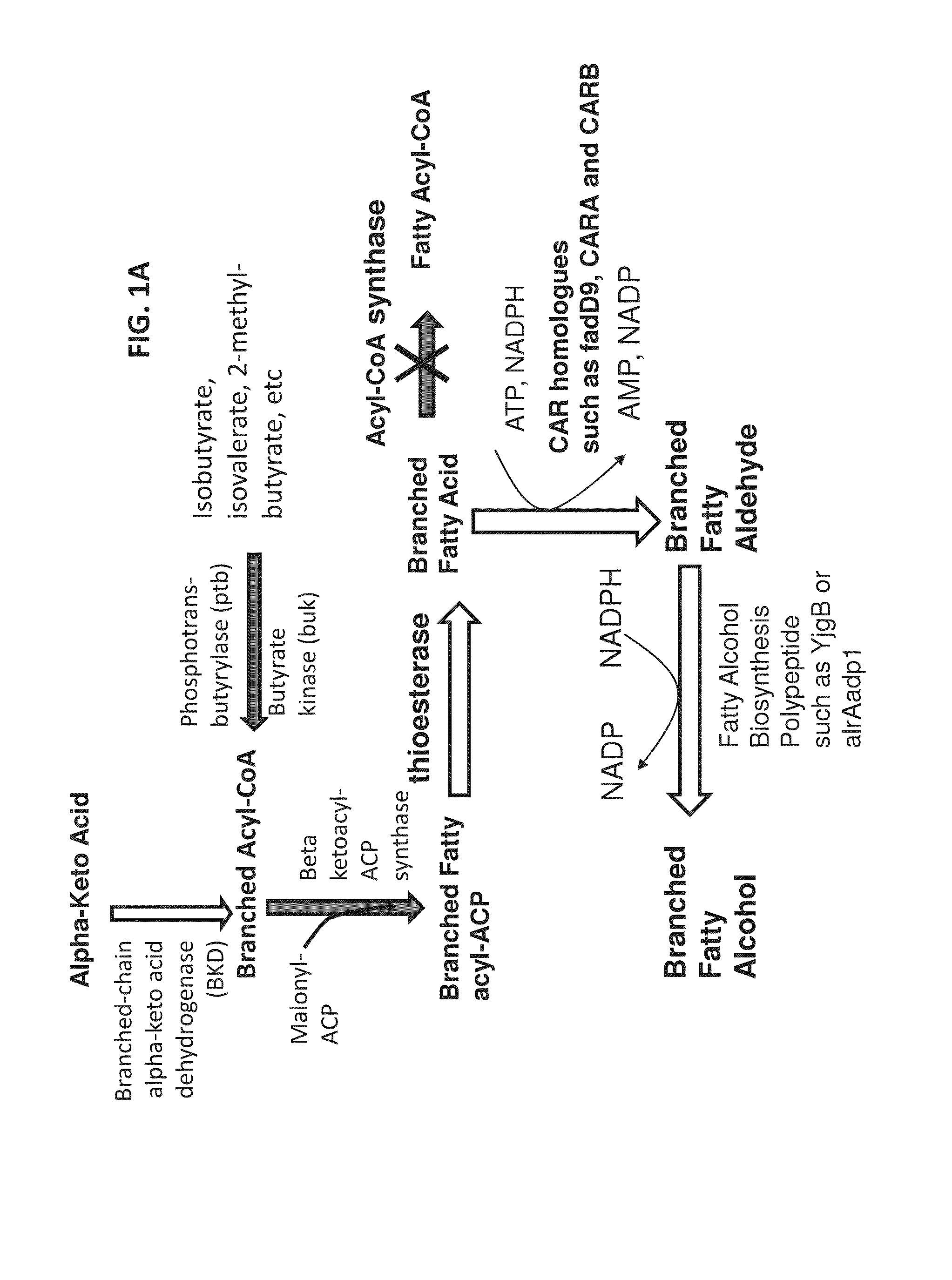
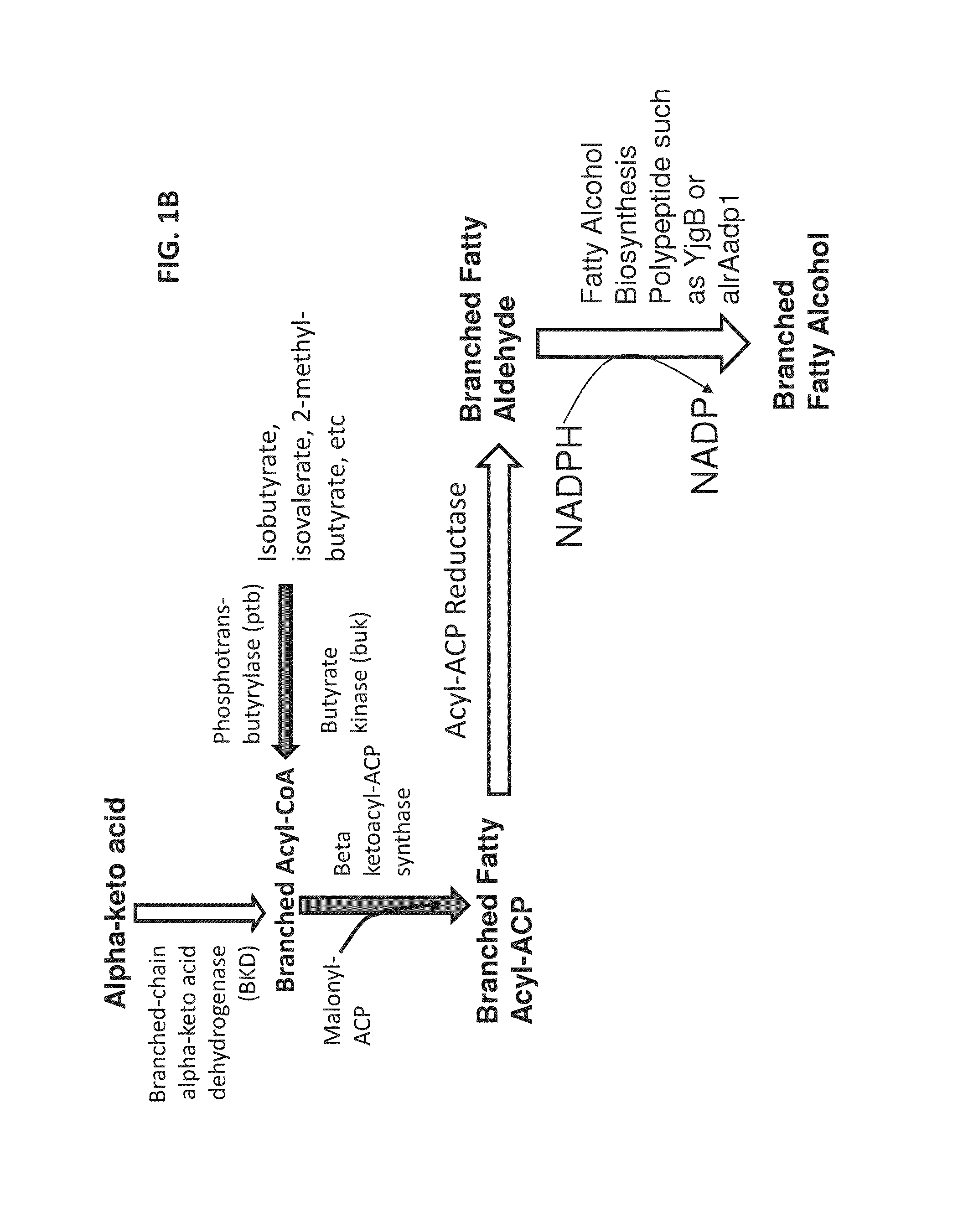
Expression of BKD Homologs and FabH in E. coli
[0489]A branched chain alpha-keto acid dehydrogenase complex from Pseudomonas putida and a FabH from Bacillus subtilis were used to generate two E. coli plasmids for expression. First, the Pseudomonas putida BKD operon was PCT-amplified from Pseudomonas putida F1 genomic DNA. The following primers were used:
[0490]
P.p.BKDFUsion_F:(SEQ ID NO: 166)5′-ATAAACCATGGATCCATGAACGAGTACGCCCC-3′P.pBKDFusion_R:(SEQ ID NO: 167)5′-CCAAGCTTCGAATTCTCAGATATGCAAGGCGTG-3′
[0491]Using these primers, Pseudomonas putida Pput—1450 (GenBank Accession No. A5W0E08), Pput—1451 (GenBank Accession No. A5W0E9), Pput—1452 (GenBank Accession No. A5W0F0), and Pput—1453 (A5W0F1) were amplified. The PCR product was then cloned into vector pGL10.173B (See, FIG. 8), a plasmid with a pBR322 backbone and a pTrc promoter to drive gene expression. The PCR product was cloned into pGL between BamHI and EcoRI restriction sites. Correct insertion of the PCR product was verified by di...
example 3
Quantification and Identification of Branched Fatty Alcohols
[0494]The instrument is an Agilent 5975B MSD system equipped with a 30 m×0.25 mm (0.10 μm film) DB-5 column. The mass spectrometer was equipped with an electron impact ionization source. Two GC / MS programs were utilized.
[0495]GC / MS program #1: The temperature of the column is held isothermal at 90° C. for 5 min, then is raised to 300° C. with a 25° C. / min ramp, and finally stays at 300° C. for 1.6 min. The total run time is 15 min. With this program, the inlet temperature is hold at 300° C. The injector is set at splitless mode. 1 μL of sample is injected for every injection. The carrier gas (helium) is released at 1.0 mL / min. The source temperature of the mass spectrometer is held at 230° C.
[0496]GC / MS program #2: The temperature of the column is held isothermal at 100° C. for 3 min, then is raised to 320° C. with 20° C. / min, and finally stays isothermal at 320° C. for 5 min. The total run time is 19 min. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com