INGAP protein involved in pancreatic islet neogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0121]This example describes the cloning and isolation of a cDNA encoding a novel, developmentally regulated, pancreatic protein.
[0122]We hypothesized that a unique locally produced factor(s) is responsible for islet cell regeneration. Using the recently developed mRNA differential display technique (5,6) to compare genes differentially expressed in cellophane wrapped (CW) versus control pancreata (CP) allowed us to identify a cDNA clone (RD19-2) which was uniquely expressed in cellophane wrapped pancreas.
[0123]A cDNA library was constructed from mRNA isolated from cellophane wrapped hamster pancreas using oligo d(T) primed synthesis, and ligation into pcDNA3 vector (Invitrogen). The number of primary recombinants in the library was 1.2×106 with an average size of 1.1 kb. The cDNA library was screened for clones of interest using high density colony plating techniques. Colonies were lifted onto nylon membranes (Schleicher & Schuell) and further digested with proteinase K (50(g / ml). ...
example 2
[0124]This example compares the sequence of INGAP to other proteins with which it shares homology.
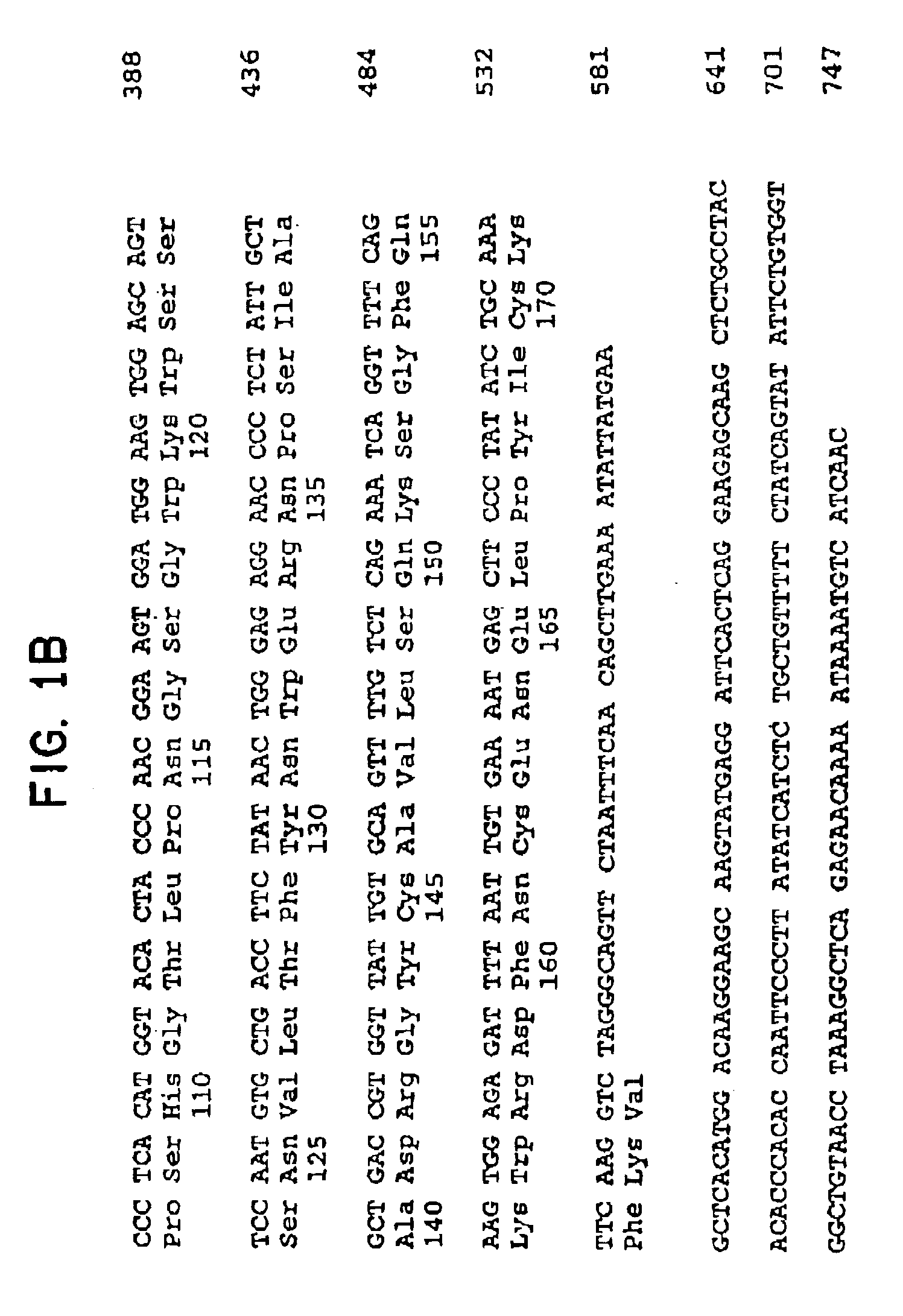
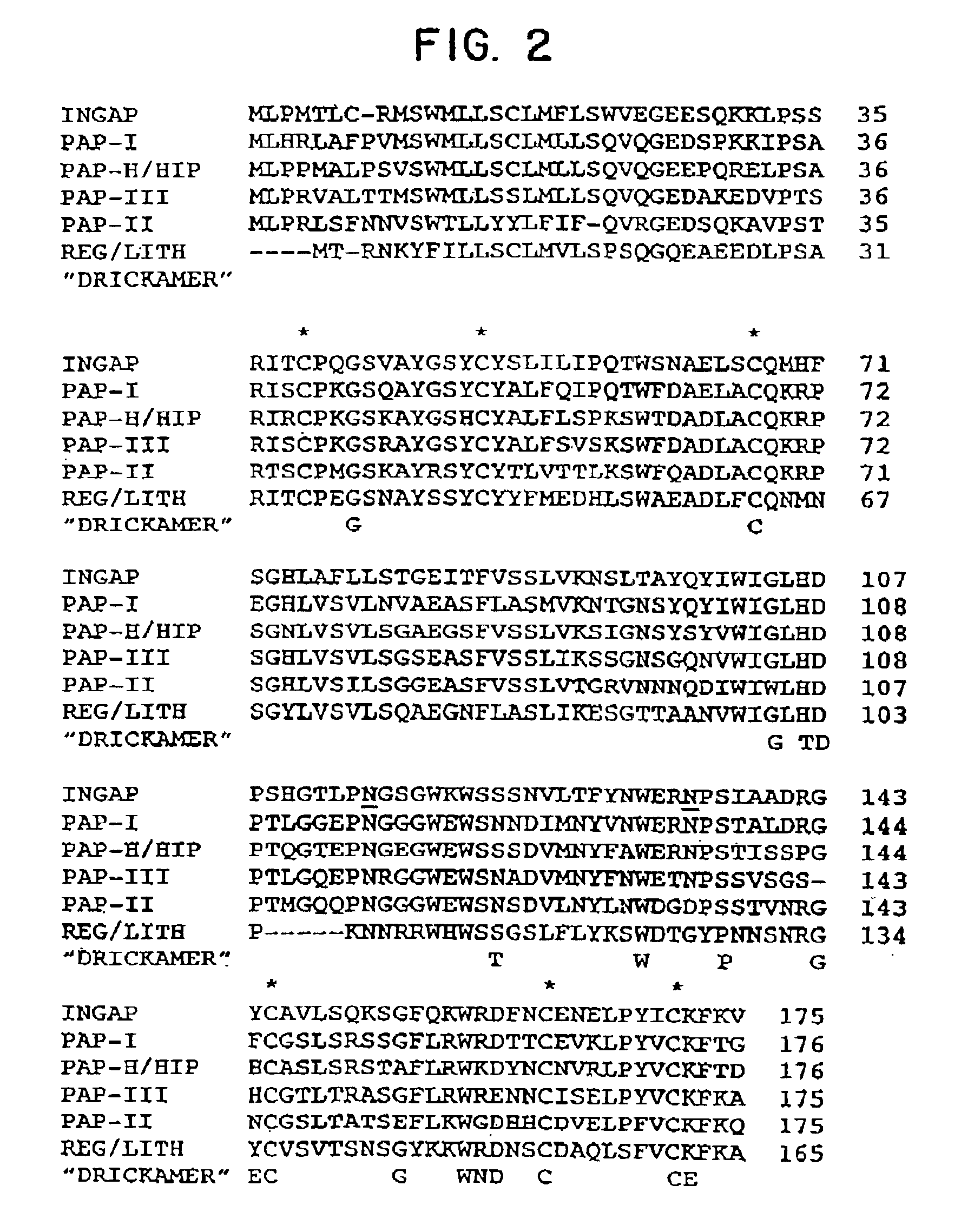
[0125]The nucleotide sequence of the hamster INGAP clone with the longest cDNA insert was determined. As shown in FIG. 1 the hamster cDNA comprises 747 nucleotides (nt), exclusive of the poly(A) tail and contains a major open reading frame encoding a 175 amino acid protein. The open reading frame is followed by a 3′-untranslated region of 206nt. A typical polyadenylation signal is present 11nt upstream of the poly(A) tail. The predicted INGAP protein shows structural homology to both the PAP / HIP family of genes which is associated with pancreatitis or liver adenocarcinoma (7-11) and the Reg / PSP / lithostatine family of genes (13,15) which has been shown to stimulate pancreatic beta-cell growth (14) and might play a role in pancreatic islet regeneration. Comparison of the nucleotide sequence and their deduced amino acids between hamster INGAP and rat PAP-I shows a high degree of homology i...
example 3
[0126]This example demonstrates the temporal expression pattern of INGAP upon cellophane-wrapping.
[0127]In order to determine the temporal expression of the INGAP gene, total RNA extracted from CP and CW pancreas was probed with the hamster INGAP cDNA clone in Northern blot analysis. A strong single transcript of 900 bp was detected (FIGS. 3A, 3B and 3C) 1 and 2 days after cellophane wrapping which disappeared by 6 through 42 days and was absent from CP. INGAP mRNA is associated with CW induced pancreatic islet neogenesis, since it is present only after CW. It is not likely that the increased expression of INGAP is associated with acute pancreatitis as is the case with the PAP family of genes. During the acute phase of pancreatitis the concentrations of most mRNAs encoding pancreatic enzymes including amylase are decreased significantly (16,18). In contrast, in the CW model of islet neogenesis in which high expression of INGAP has been detected, amylase gene expression was simultane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com