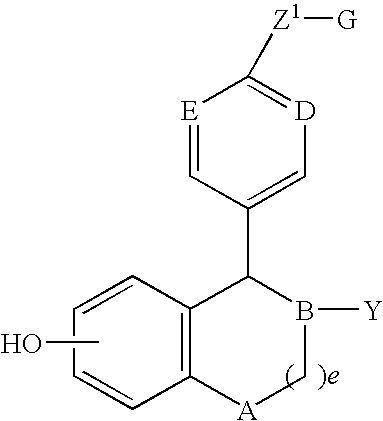
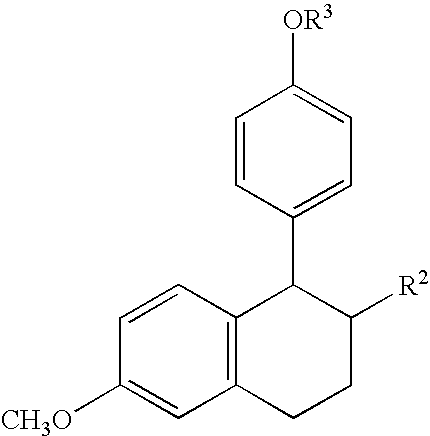
5-Substituted-6-cyclic-5,6,7,8-tetrahydronaphthalen 2-ol compounds which are useful for treating osteoporosis
a technology of tetrahydronaphthalen and substituted cyclic, which is applied in the direction of drug compositions, extracellular fluid disorders, metabolic disorders, etc., can solve the problems of increasing the risk of osteoporosis, the elderly are at the greatest risk of osteoporosis, and it is not clinically advisable to treat osteoporosis in intact women with fully active estrogens for prolonged periods. , to achieve the effect of reducing prostate weight, reducing bone strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cis-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phenyl]-5,6,7,8-tetrahydronaphthalen-2-ol
Step A
[0161]cis-1-{2-[4-(6-Methoxy-2-phenyl-1,2,3,4-tetrahydronaphthalen-1-yl)phenoxy]ethyl}pyrrolidine. A solution of 1-{2-[4-(6-methoxy-2-phenyl-3,4-dihydronaphthalen-1-yl)phenoxy]ethyl}pyrrolidine hydrochloride (nafoxidene hydrochloride) (1.0 g, 2.16 mmol) in 20 mL of absolute ethanol containing 1.0 g of palladium hydroxide on carbon was hydrogenated at 60 psi at 20° C. for 19 hr. Filtration and evaporation provided 863 mg (93%) of cis-1-{2-[4-(6-methoxy-2-phenyl-1,2,3,4-tetrahydronaphthalen-1-yl)phenoxy]ethyl}pyrrolidine: 1H-NMR (CDCl3): δ 3.50-3.80 (m, 3H), 3.85 (s, 3H), 4.20-4.40 (m, 3H), 6.80-7.00 (m, 3H); MS 428 (P++1).
Step B
[0162]To a solution of 400 mg (0.94 mmol) of the product from Step A in 25 ml of methylene chloride at 0° C. was added, dropwise with stirring, 4.7 ml (4.7 mmol) of a 1.0 M solution of boron tribromide in methylene chloride. After 3 hours at room temperature, the reaction...
example 2
Trans-6-phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)phenyl]-5,6,7,8-tetrahydronaphthalen-2-ol
Step A
[0169]To a solution of cis-1-{2-[4-(6-methoxy-2-phenyl-1,2,3,4-tetrahydronaphthalen-1-yl)phenoxy]ethyl}pyrrolidine (500 mg, 1.1 7 mmol) in 10 ml of dimethyl sulfoxide at 0° C. was added slowly 4.7 ml (11.7 mmol) of 2.5 M n-butyl lithium in hexane. The reaction was allowed to warm to 20° C. and was stirred for 19 hrs. After addition of water and extraction with ether, the organic layers were combined, dried over magnesium sulfate, filtered and concentrated to dryness to yield 363 mg (73%) of the trans-6-methoxydihydronaphthalene. 1H-NMR (CDCl3): δ 3.45 (m, 2H), 3.82, (s, 3H), 4.06 (d, 1H), 4.45 (m, 2H), 6.80 (d, 2H).
Step B
[0170]Using the boron tribromide deprotection procedure described in Example 1 Step B, 363 mg (0.85 mmol) of the product of Step A was converted to 240 mg (68%) of the title compound. 1H-NMR (CDCl3): δ 4.02 (d, 1H), 4.45 (m, 2H), 7.00 (d, 2H). The corresponding hydrochloride...
example 3
1-(4′-Pyrrolidinoethoxyphenyl-2-(4″-hydroxyphenyl)-6-hydroxy-1,2,3,4-tetrahydroisoquinoline hydrochloride
Step A
[0171]3-Methoxyphenylacet-4′-methoxyanilide. A solution of 20.0 g (0.120 mole) of 3-methoxyphenylacetic acid and 40 ml (65.3 g, 0.549 mole) of thionyl chloride in 100 ml of benzene was heated at reflux for 2 hours and evaporated to dryness to afford the corresponding acid chloride (assume 0.120 mole). The acid chloride was slurried in 50 ml of ether and added to a mixture of 4-methoxyaniline in 100 ml of ether at 0° C. After stirring at 20° C. overnight, the slurry was filtered to afford a solid which was washed with water, 5.5% aq HCl, water, and ether. Subsequent drying over P2O5 in vacuo for 4 hr. yielded 19.7 g (60%) of the title substance as a white solid. 1H-NMR (CDCl3): δ 3.70 (s, 2H), 3.77 (s, 3H), 3.81 (s, 3H).
Step B
[0172]N-(4-Methoxyphenyl)-2′-(3″-methoxyphenethylamine)hydrochloride: A slurry of 19.6 g (0.072 mol) of the product of Step A and 6.04 g (0.159 mol) of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com