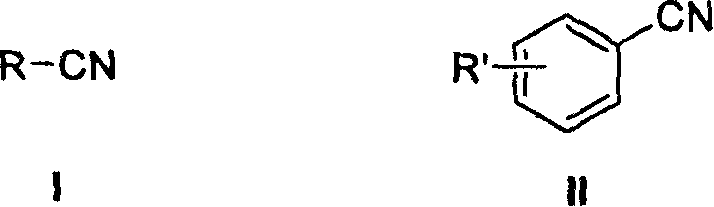Nitrile reducing process to prepare amine
A technology based on primary amines and hydrocarbon groups, which is applied in the field of reducing nitriles to prepare amines, achieving the effects of low cost, fast speed and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: This embodiment prepares phenylethylamine, raw material is benzyl nitrile:
[0016] Add 5-80mmol KBH to the round bottom flask 4 , put a magnetic stirring bar, add 0-80mL water, carefully add 1-80mmol Raney nickel, then add 5-80mL ethanol and 1.16mL (10mmol) phenylacetonitrile, and stir at -15~70°C. After 45 minutes, the Raney nickel was filtered off, and the filtrate was evaporated to remove ethanol with a rotary evaporator, and 50 mL of ethyl acetate was added to dissolve the product, transferred to a 125 mL separating funnel, washed with water for 3 to 4 times, and the organic phase was transferred to a 100 mL round bottom flask. Add anhydrous Na 2 SO 4 Dry overnight, filter off the desiccant, evaporate ethyl acetate with a rotary evaporator, and use aluminum oxide column chromatography (developing solvent is dichloromethane: methanol 20: 1) to obtain light yellow liquid phenethylamine. 92%.
Embodiment 2
[0017] Embodiment 2: This embodiment prepares p-methoxyphenethylamine, raw material is p-methoxyphenylacetonitrile
[0018] Add 5-80mmol KBH to the round bottom flask 4 , put a magnetic stirring bar, add 0-80mL water, carefully add 1-80mmol Raney nickel, then add 5-80mL ethanol and 1.36mL (10mmol) p-methoxyphenylacetonitrile, and stir at -15~70°C. After 45 minutes, the Raney nickel was filtered off, and the filtrate was evaporated to remove ethanol with a rotary evaporator, and 50 mL of ethyl acetate was added to dissolve the product, transferred to a 125 mL separating funnel, washed with water for 3 to 4 times, and the organic phase was transferred to a 100 mL round bottom flask. Add anhydrous Na 2 SO 4 Dry overnight, filter off the desiccant, evaporate ethyl acetate with a rotary evaporator, and use aluminum oxide column chromatography (developing solvent is dichloromethane:methanol 20:1) to obtain a colorless liquid p-methoxyphenethyl Amine, 86% yield.
Embodiment 3
[0019] Embodiment 3: This embodiment prepares p-chlorophenethylamine, raw material is p-chlorophenylacetonitrile
[0020] Add 5-80mmol KBH to the round bottom flask 4 , put a magnetic stirring bar, add 0-80mL water, carefully add 1-80mmol Raney nickel, then add 5-80mL ethanol and 1.51g (10mmol) p-chlorophenylacetonitrile, and stir at -15~70°C. After 45 minutes, the Raney nickel was filtered off, and the filtrate was evaporated to remove ethanol with a rotary evaporator, and 50 mL of ethyl acetate was added to dissolve the product, transferred to a 125 mL separating funnel, washed with water for 3 to 4 times, and the organic phase was transferred to a 100 mL round bottom flask. Add anhydrous Na 2 SO 4 Dry overnight, filter off the desiccant, evaporate ethyl acetate with a rotary evaporator, and use aluminum oxide column chromatography (developing solvent is dichloromethane:methanol 20:1) to obtain a colorless liquid p-methoxyphenethyl Amine, 90% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com