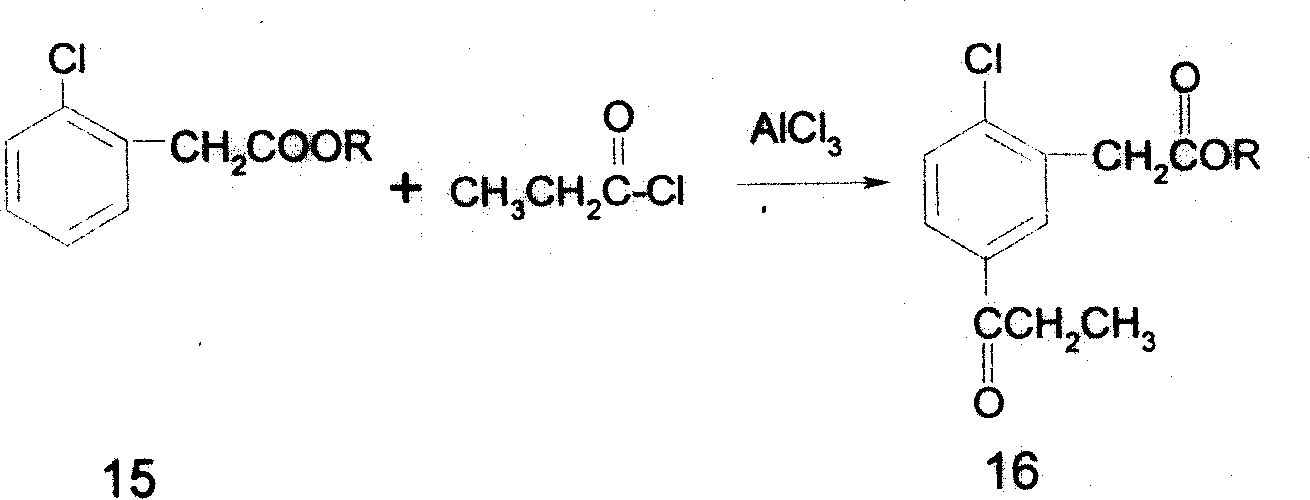
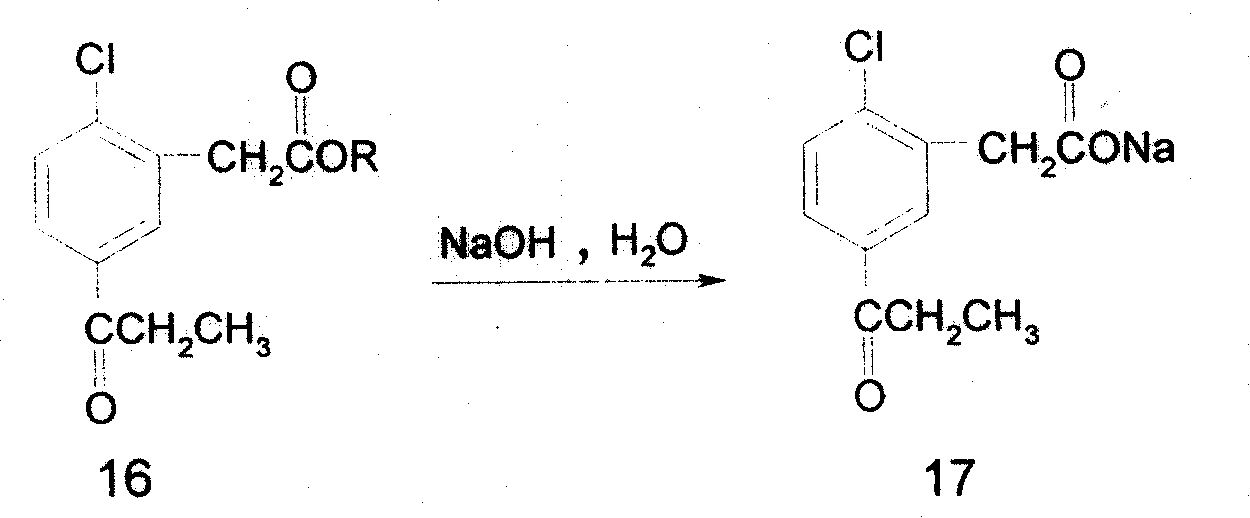
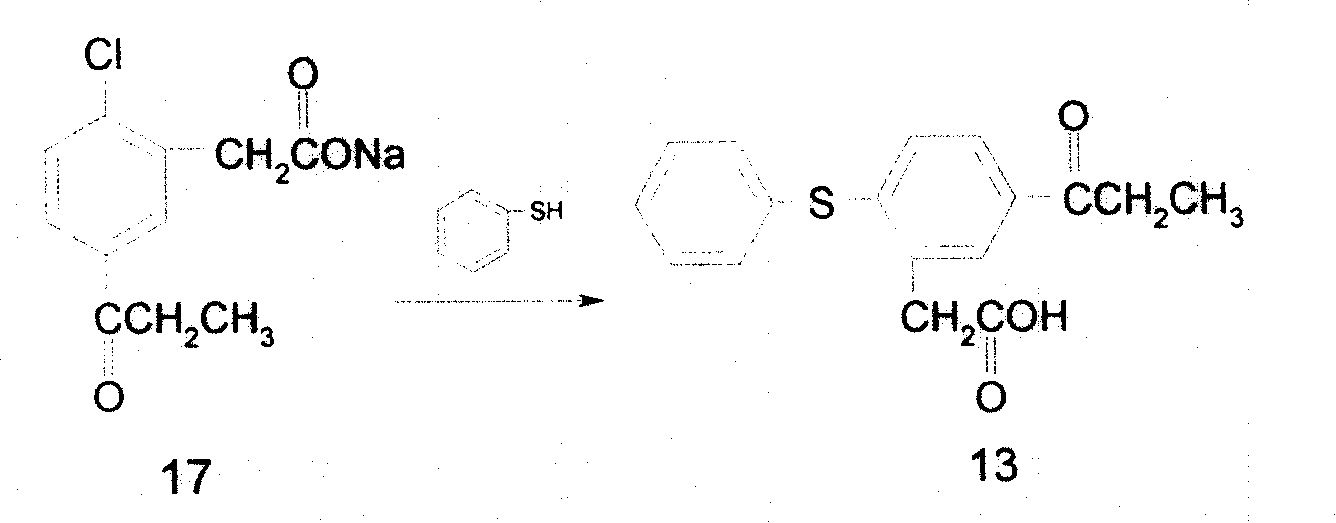
Preparing process of 5-propionyl-2-thiophenyl phenylacetate
A technology of phenylthiophenylacetate and phenylacetate, which is applied in the field of preparation of 5-propionyl-2-phenylthiophenylacetate and can solve the problem of troublesome solid separation, incomplete reaction and low yield And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] 1, the preparation of methyl o-chlorophenylacetate (15)
[0092] Raw material specification and feeding amount
[0093] Raw material (molecular weight)
Specification
Feeding amount
o-Chlorophenylacetic acid (170.5)
Industrial first-class product, white with acetic acid smell
Color powder.MP95~97℃
10Kg
Methanol(32)
Colorless, transparent, flammable and volatile polar liquid
Body ≥ 99%
19Kg
Concentrated sulfuric acid (98)
Colorless transparent oily liquid, strong corrosion,
Content≥98%
1.14Kg
[0094] Feeding ratio (molar ratio):
[0095] O-chlorophenylacetic acid: methanol: concentrated sulfuric acid = 1: 10.12: 0.20
[0096] Add o-chlorophenylacetic acid and methanol to the reaction kettle respectively, stir, add concentrated sulfuric acid dropwise under cooling, heat up and reflux for 10 hours, and remove the solvent. Add water, separate layers, neutralize the or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com