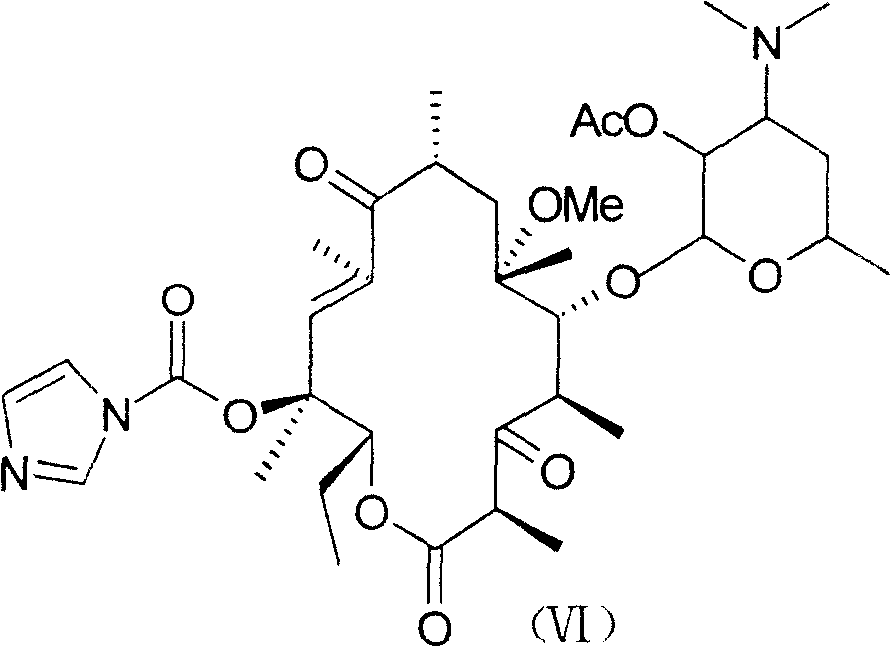
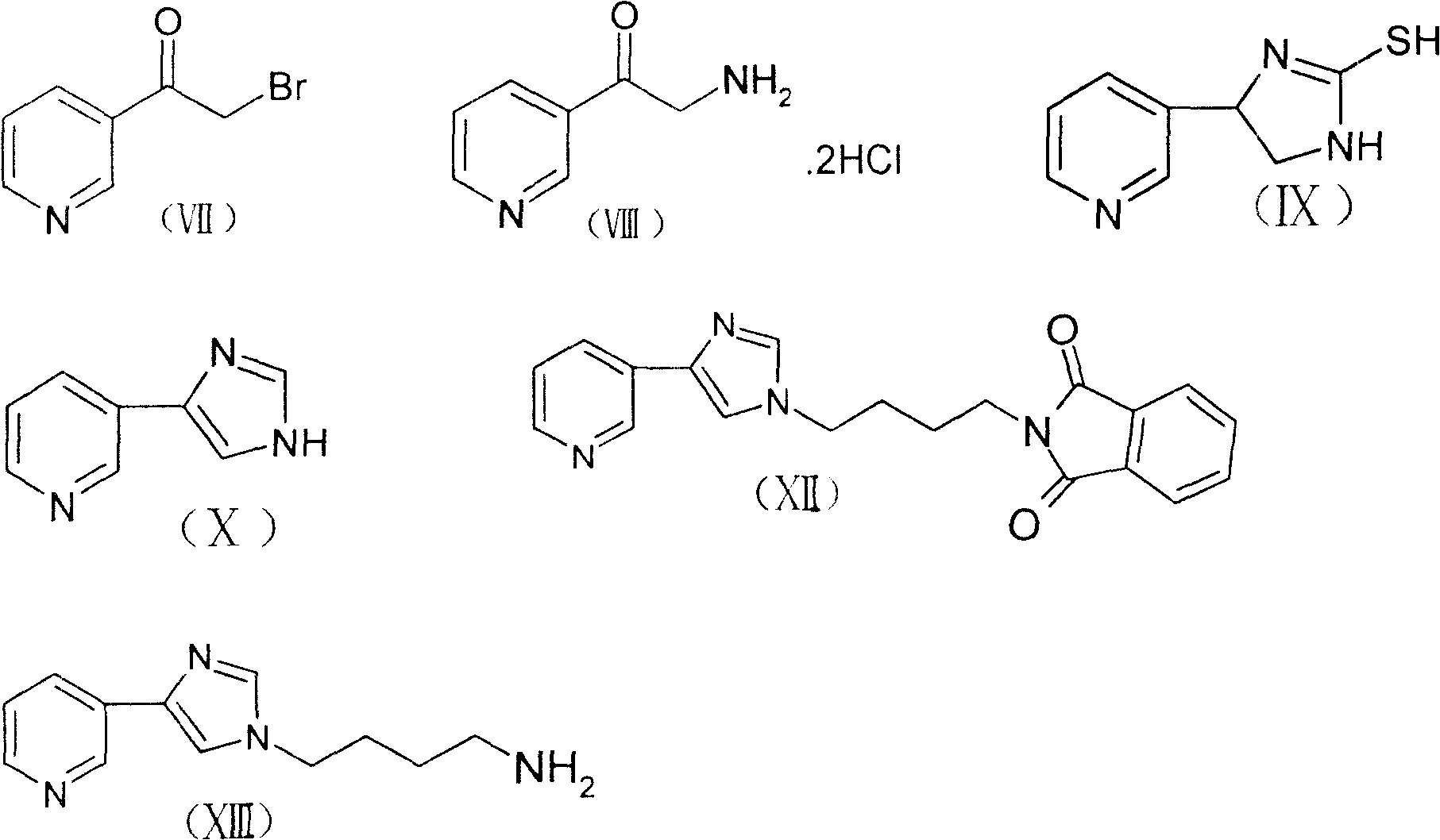
Method for preparing macrolides half-synthesized antibiotics telithromycin
A technology of macrolides and telithromycin, applied in the preparation of sugar derivatives, chemical instruments and methods, antibacterial drugs, etc., can solve the problems of low reaction yield, high price, high risk, etc., and achieve safety Good performance and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
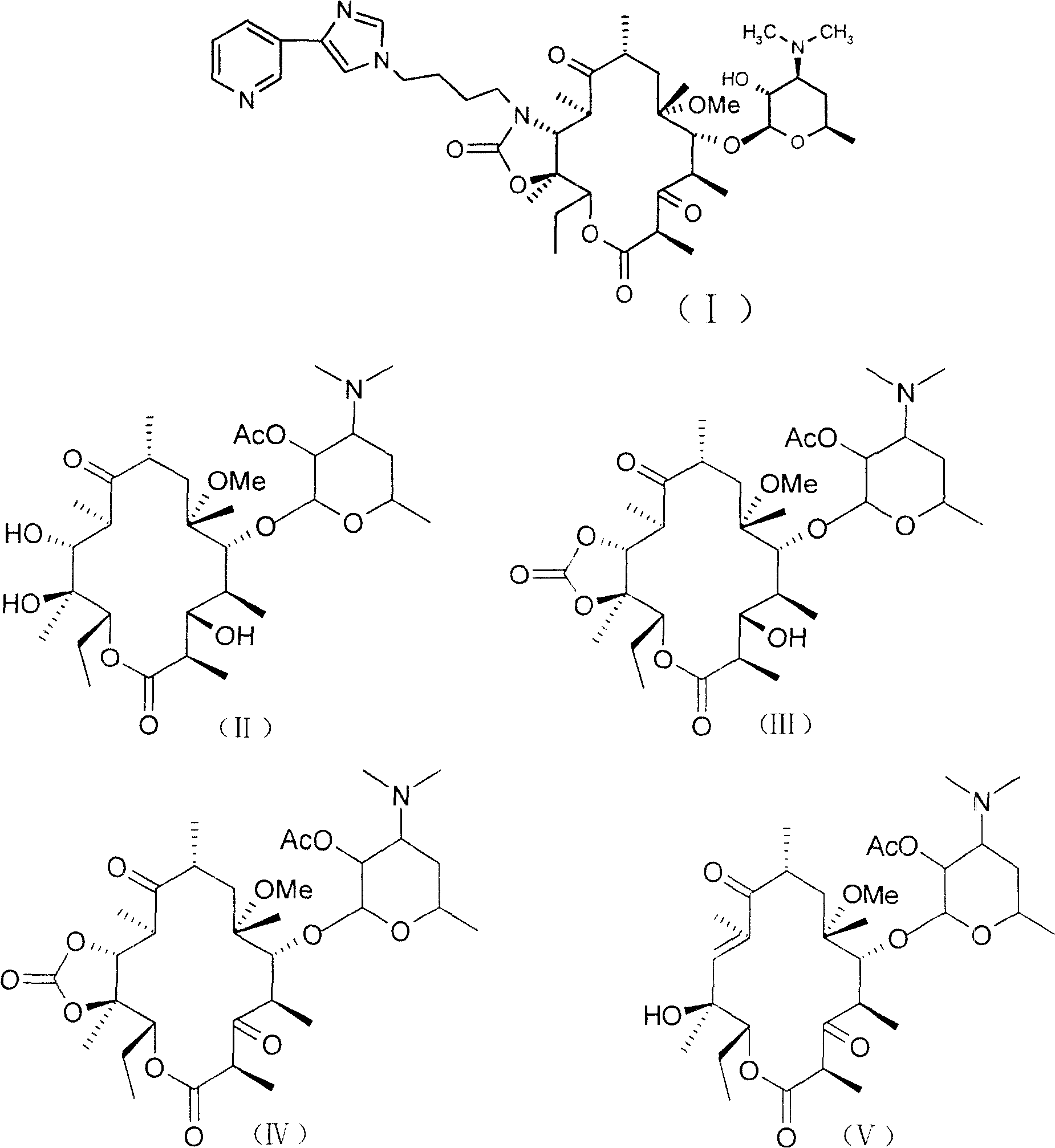
[0032] 3-Des[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribohexopyranosyl)oxy]-6-O-methylerythromycin
[0033] Disperse 60.0g (80.4mmol) of 6-O-methylerythromycin (clarithromycin) in 1000ml of 1M hydrochloric acid, stir at room temperature for about 5 hours, adjust the pH to 8-9 with concentrated ammonia water, add solid sodium chloride to make The solution was saturated, filtered and washed with water to obtain a white solid, which was recrystallized from acetone and petroleum ether (60-90°C) to obtain 40.2g, yield 84.9%, mp 236-240°C.
[0034] IR (KBr) 1690, 1734cm -1
[0035] 1 HNMR (CDCl 3 )δ5.18 (dd, 1H, C-13H, J=13.5Hz), 2.97 (s, 3H, C-6OCH 3 ), 2.25(s, 6H, N(CH 3 ) 2 )ppm
[0036] MS(ESI) m / e: 590(M+H) +
Embodiment 2
[0038] 2'-O-acetyl-3-de[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribohexopyranosyl)oxy]-6- O-methylerythromycin (II):
[0039] 3-de[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribohexopyranosyl)oxy]-6-O-methylerythromycin ( Obtained from Example 1) 40.0g (68mmol) was dissolved in 400ml of dichloromethane and 11.6ml (80.4mmol) of triethylamine, and 13.44ml (141.12mmol) of acetic anhydride was added dropwise under ice bath, and the ice bath was removed after the addition , Reaction at room temperature for 3.5h. Add saturated NaHCO 3 150ml of aqueous solution was extracted with chloroform, dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure. The crude product was recrystallized from ethyl acetate and petroleum ether (15:1) to obtain 39g of white soft solid, yield 90.9%, mp 156- 160°C.
[0040] IR (KBr) 1690, 1734, 1741cm -1
[0041] 1 HNMR (CDCl 3 ) δ 4.85 (dd, 1H, C-2'CHOAC, J=18Hz), 3.04 (s, 3H, C-6OCH 3 ), 2.36(s, 6H, N(CH 3 ) ...
Embodiment 3
[0044] 2'-O-acetyl-3-de[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribohexopyranosyl)oxy]-6- O-methyl-11,12-carbonate erythromycin (III)
[0045] Compound (II) (obtained by Example 2) 20 grams (31.7mmol) was dissolved in 200ml toluene, added diethyl carbonate 40g (450.5mmol), anhydrous potassium carbonate 40g (290mmol), room temperature reaction 9 hours, washed three times , anhydrous MgSO 4 After drying, the solvent was removed under reduced pressure to obtain an off-white solid, which was recrystallized from acetone solution to obtain 15.6 g of pure product with a yield of 75%, m.p.92-93°C.
[0046] IR (KBr) 3540, 1814, 1741, 1715cm -1
[0047] 1 HNMR (CDCl 3 )δ5.0(dd,1H,H 13 , J=2.4, 10.2Hz), 2.64(s, 3H, 6-O-CH 3 ), 2.24(s, 6H, N(CH 3 ) 2 ), 2.04 (s, 3H, 2'OCOCH 3 )ppm
[0048] MS(ESI) m / e: 658(M+H) +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com