Hydrochloric acid cefetamet pivoxil dispersible tablet and method for preparing the same
A technology of ceftazidime pivoxil hydrochloride and dispersible tablets, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. It can solve the problems of strong viscosity, difficulty in making granules, and poor chemical properties. Stability and other issues, to achieve the effect of improving dissolution and dispersion performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
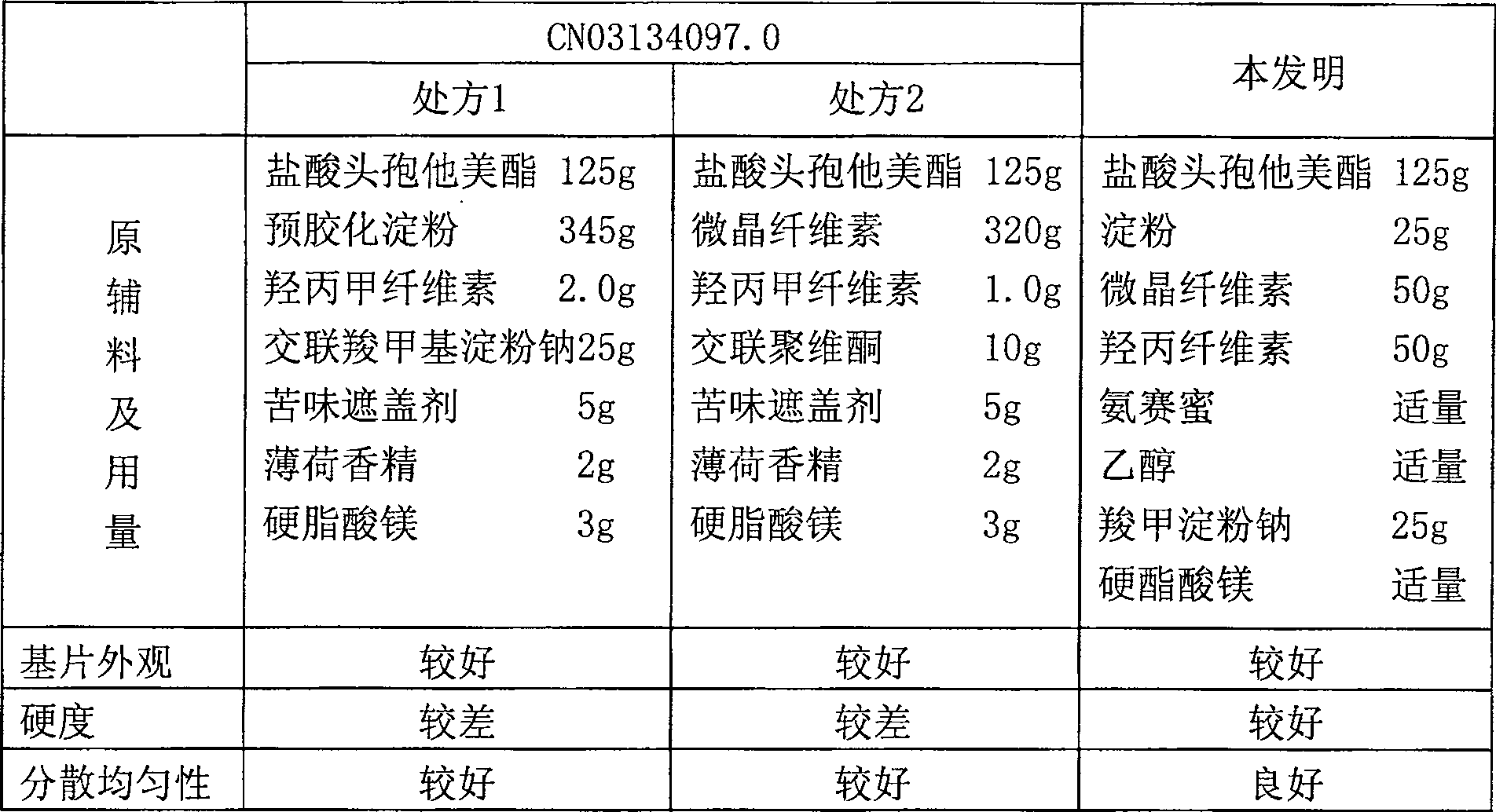
[0069] prescription:
[0070]
[0071] Preparation Process:
[0072] 1) Prepare materials
[0073] Ceftazidime hydrochloride was pulverized, passed through a 100-mesh sieve, starch, microcrystalline cellulose, hydroxypropyl cellulose, acesulfame potassium and carboxymethyl starch sodium were respectively pulverized, and passed through a 120-mesh sieve to obtain spare raw materials and auxiliary materials;
[0074] 2) Humidification granules
[0075] Weigh the above-mentioned spare ceftazidime hydrochloride, starch, microcrystalline cellulose, acesulfame potassium and hyprolose in the prescribed amount, put them into a high-speed mixing granulator, seal and dry-mix at high speed for 10 minutes, and then add an appropriate amount of ethanol Wet mixing for 3 minutes, after wet mixing, wet mixing and cutting for 2 minutes, releasing granules to obtain wet granules;
[0076] 3) Make dry granules
[0077] Transfer the wet granules prepared in the previous step into a boiling ...
Embodiment 2
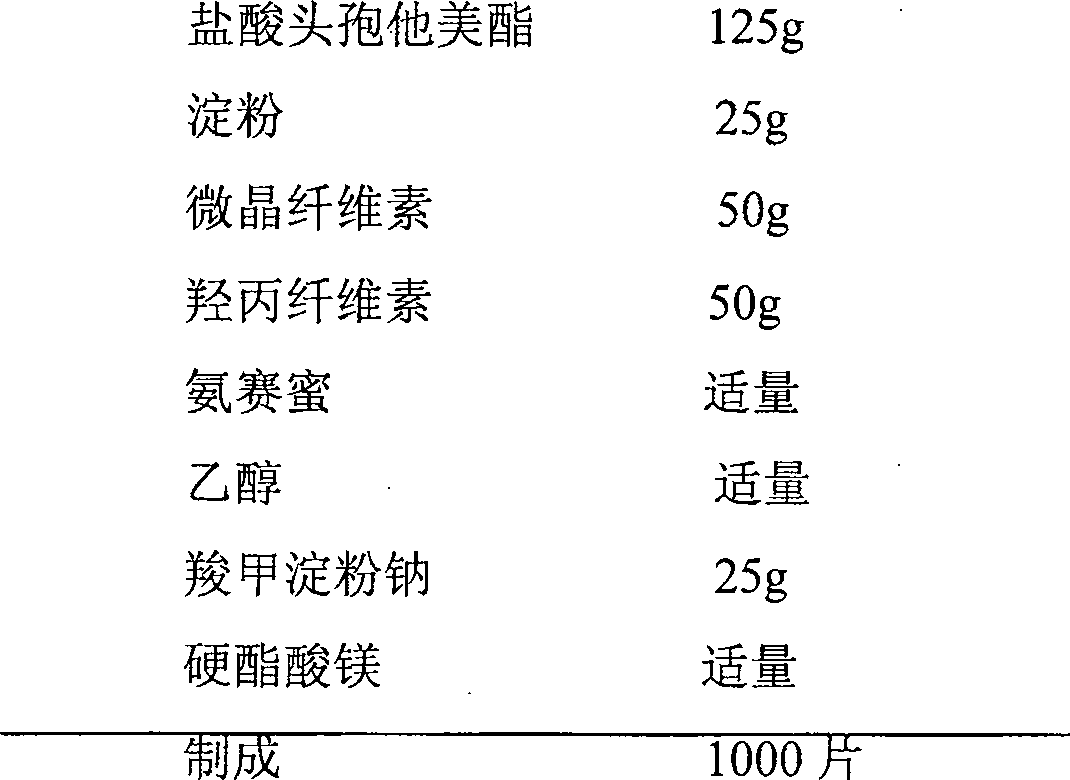
[0081] prescription:
[0082]
[0083] Preparation Process:
[0084] 1) Prepare materials
[0085] Ceftazidime hydrochloride was pulverized, passed through a 100-mesh sieve, starch, microcrystalline cellulose, hydroxypropyl cellulose, acesulfame potassium and carboxymethyl starch sodium were respectively pulverized, and passed through a 120-mesh sieve to obtain spare raw materials and auxiliary materials;
[0086] 2) Humidification granules
[0087] Weigh the above-mentioned spare ceftazidime hydrochloride, starch, microcrystalline cellulose, acesulfame potassium and hyprolose in the prescribed amount, put them into a high-speed mixing granulator, seal and dry-mix at high speed for 5 minutes, and then add an appropriate amount of ethanol Wet mixing for 1 minute, after wet mixing, wet mixing and cutting for 3 minutes, releasing granules to obtain wet granules;
[0088] 3) Make dry granules
[0089] Transfer the wet granules prepared in the previous step to a boiling drye...
Embodiment 3
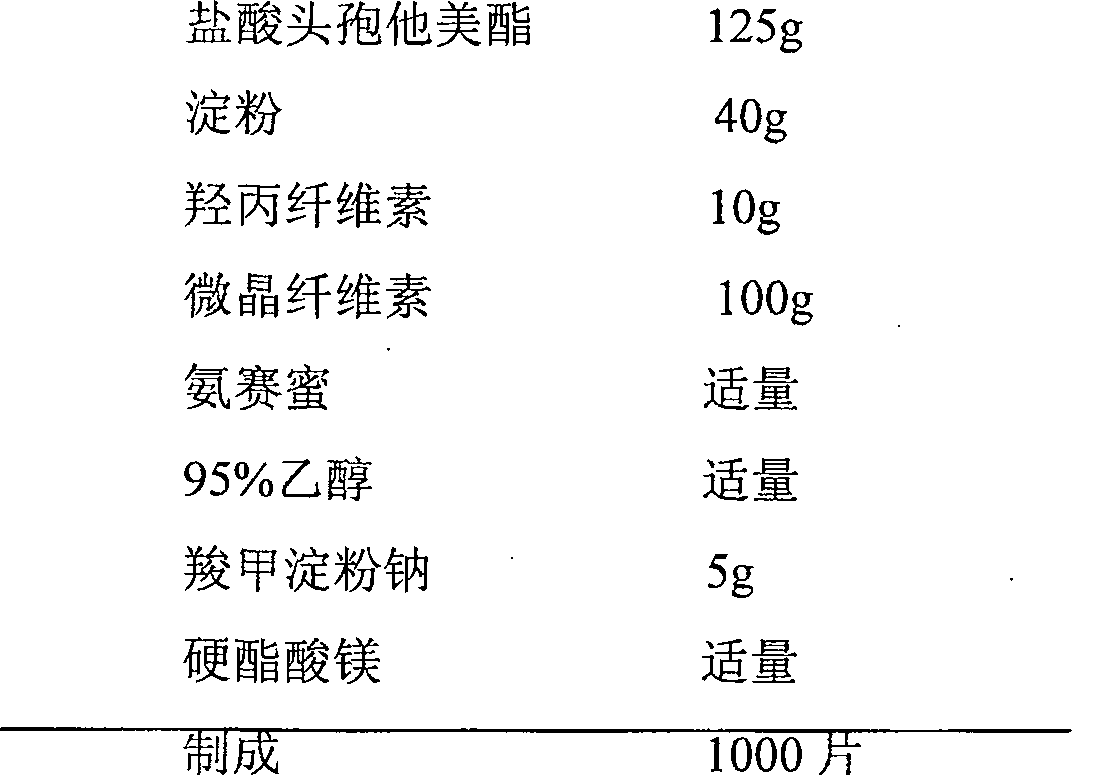
[0093] prescription:
[0094]
[0095]
[0096] Preparation Process:
[0097] 1) Prepare materials
[0098] Ceftazidime hydrochloride was pulverized, passed through a 100-mesh sieve, starch, microcrystalline cellulose, hydroxypropyl cellulose, acesulfame potassium and carboxymethyl starch sodium were respectively pulverized, and passed through a 120-mesh sieve to obtain spare raw materials and auxiliary materials;
[0099] 2) Humidification granules
[0100] Weigh the above-mentioned standby ceftazidime hydrochloride, starch, microcrystalline cellulose, acesulfame potassium and hyprolose in the prescribed amount, put them into a high-speed mixing granulator, seal and dry-mix at high speed for 15 minutes, and then add an appropriate amount of ethanol Wet mixing for 5 minutes, after wet mixing, wet mixing and cutting for 1 minute, releasing the granules to obtain wet granules;
[0101] 3) Make dry granules
[0102] Transfer the wet granules prepared in the previous ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com