Spectrophotometry for testing activity of pyruvic acid dehydrogenase system
A pyruvate dehydrogenase, activity technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, color/spectral property measurement, etc., can solve the problems of no spectrophotometry, unstable radioactive substances, environmental pollution, etc. Achieve the effect of small standard error, high sensitivity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
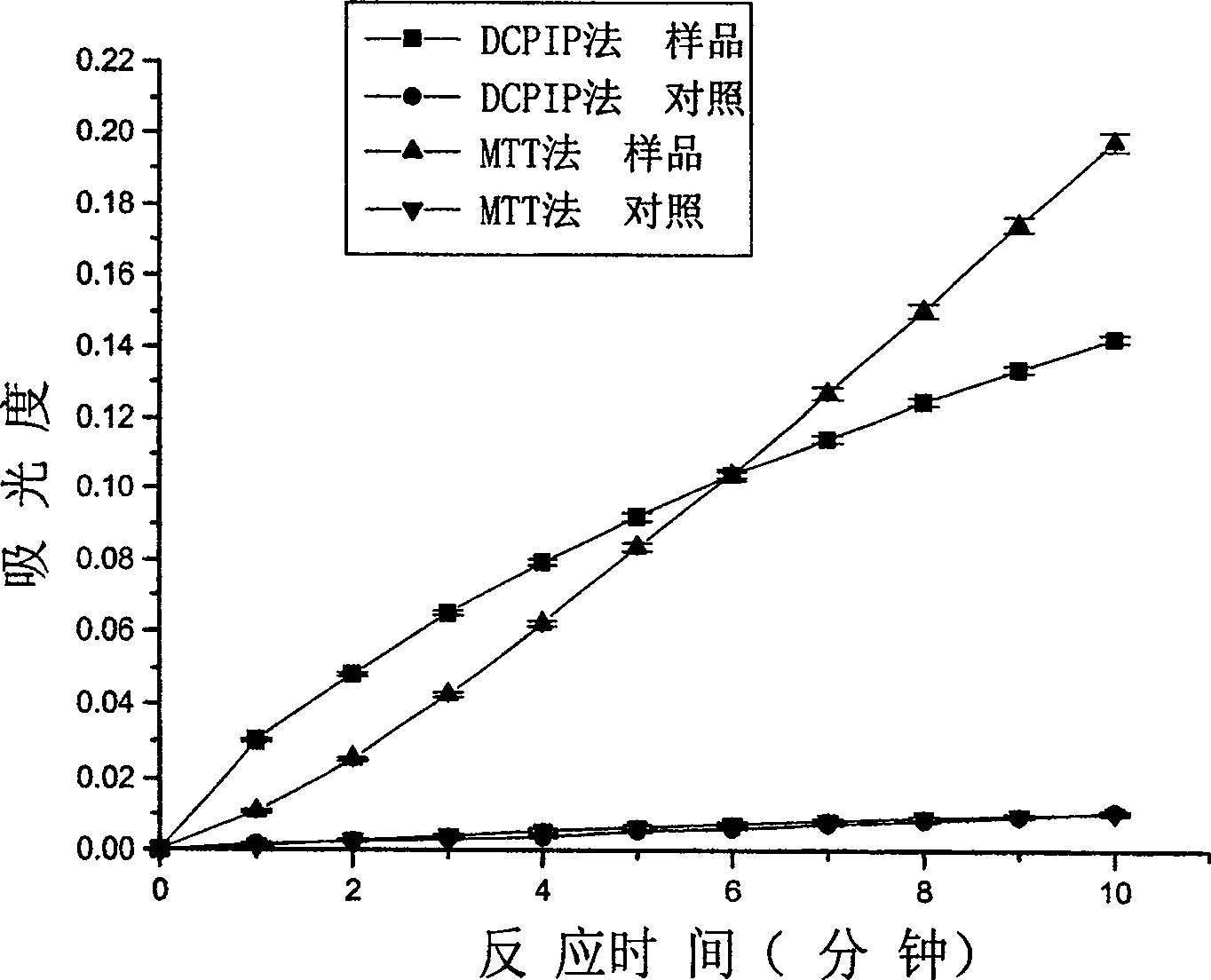
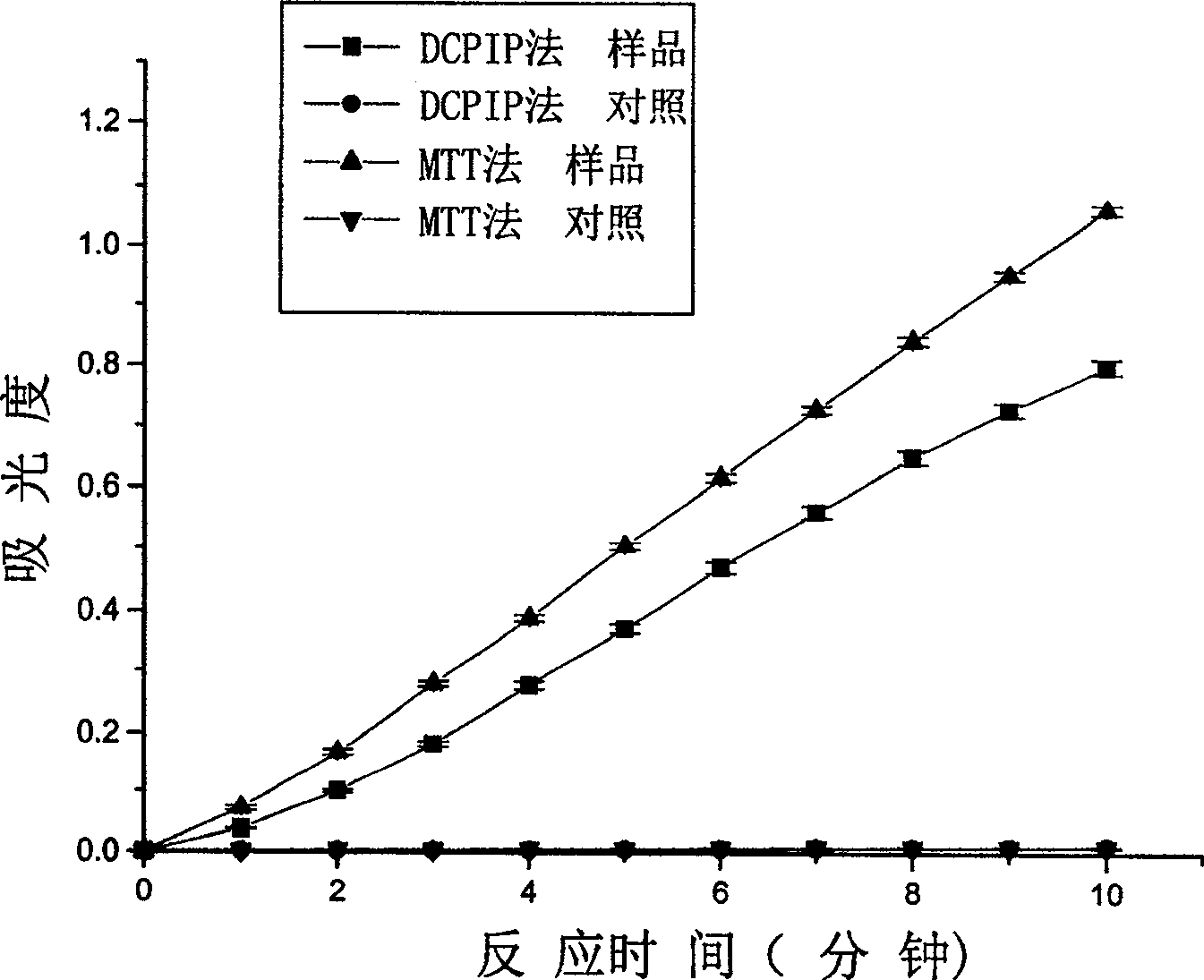
[0018] Embodiment 1: MTT method and DCPIP method measure the comparison of the enzyme activity of pyruvate dehydrogenation in the enzyme of lactic acid bacteria pyruvate dehydrogenation and pig heart pyruvate dehydrogenase complex (PDC)
[0019] Step 1: Enzyme pretreatment--lactobacillus pyruvate dehydrogenase (PDH) preparation, PDH powder is dissolved and diluted to 1 mg protein / ml. The porcine heart pyruvate dehydrogenase complex was diluted to 1 mg protein / ml with 50 mM potassium phosphate buffer pH 7.1 containing 20% glycerol. Store at -20°C after aliquoting.
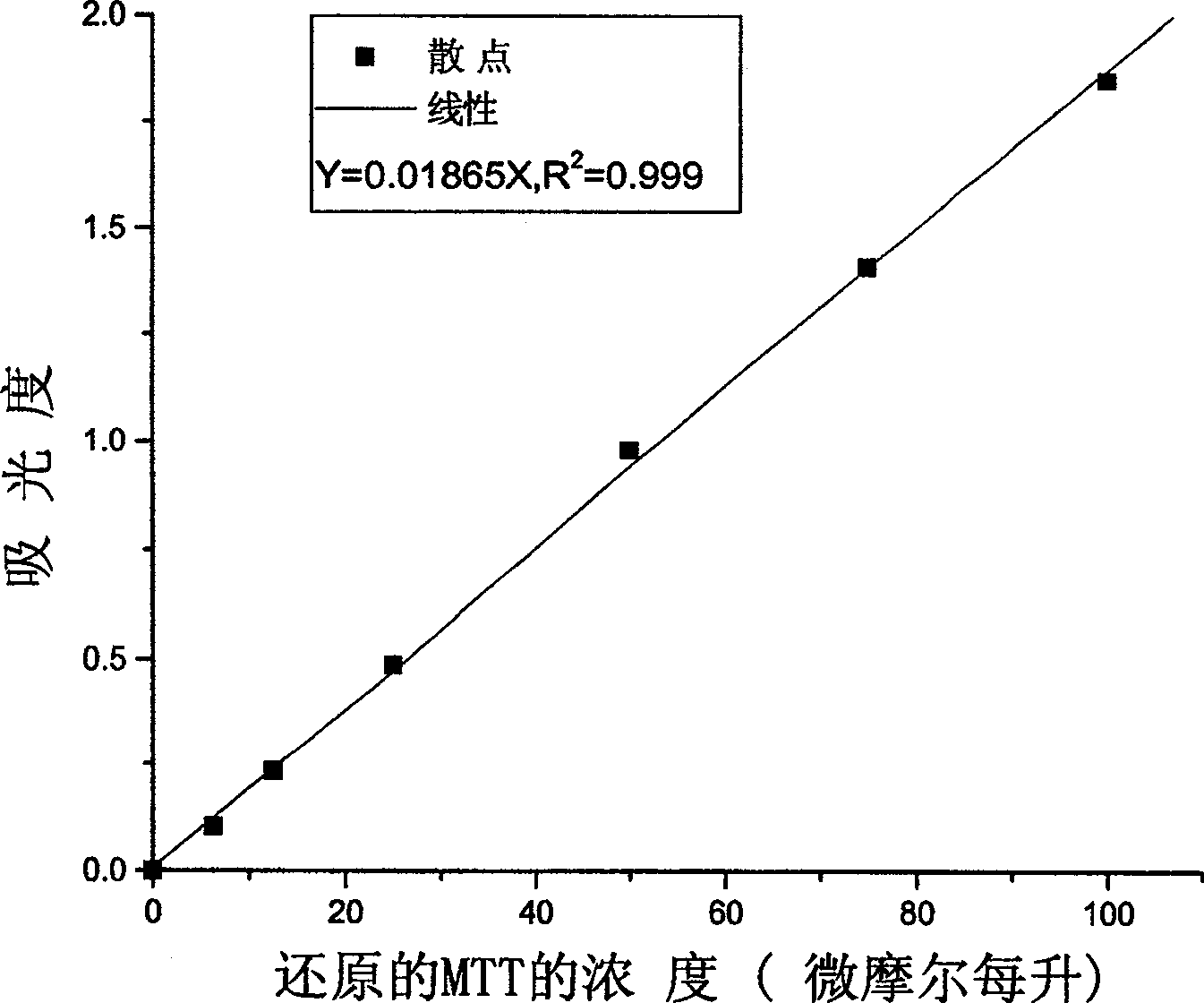
[0020] The second step: the preparation of the MTT method reaction mixture: the reaction mixture contains 50 mmol pH7.1 potassium phosphate buffer, 1 mmol / L MgCl 2 , 0.2 mmol / L thiamine pyrophosphate, 0.5 mmol / L MTT, 6.5 mmol / L phenazine dimethyl sulfate and 2.0 mmol / L sodium pyruvate were used as substrates for the reaction. The DCPIP method was modified with reference to the reaction system used by Nemeria e...
Embodiment 2
[0025] Embodiment 2: Determination of inhibition rate of inhibitor to pyruvate dehydrogenase activity
[0026] Step 1: Enzyme pretreatment--lactobacillus pyruvate dehydrogenase (PDH) preparation, PDH powder is dissolved with 50 millimolar pH7.1 potassium phosphate buffer solution containing 20% glycerol and diluted to a concentration of 1 mg protein / ml.
[0027] The second step: with the second step of embodiment 1
[0028] The third step: the enzyme and the inhibitor IIM-1 (IIM-1: 1-(2,4-disubstituted phenoxyacetoxyalkylphosphonic acid monosodium salt compound) with a concentration of 100ppm) were incubated at 30°C for 30 -60 minutes
[0029] Step 4: At room temperature, add enzyme or enzyme and inhibitor to the reaction mixture to initiate the reaction, and measure the relationship between the absorbance of the reaction mixture and time, the results are shown in Figure 4.
[0030] In Fig. 4: pure enzyme is the complete reaction system adding enzyme solution to start the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com