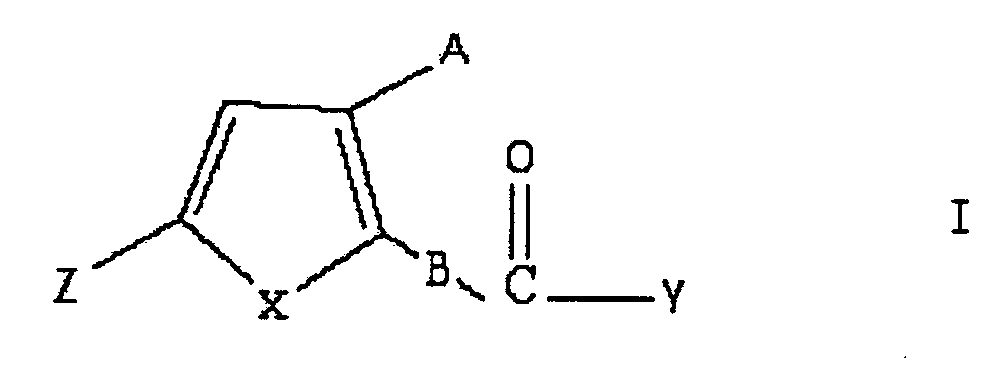
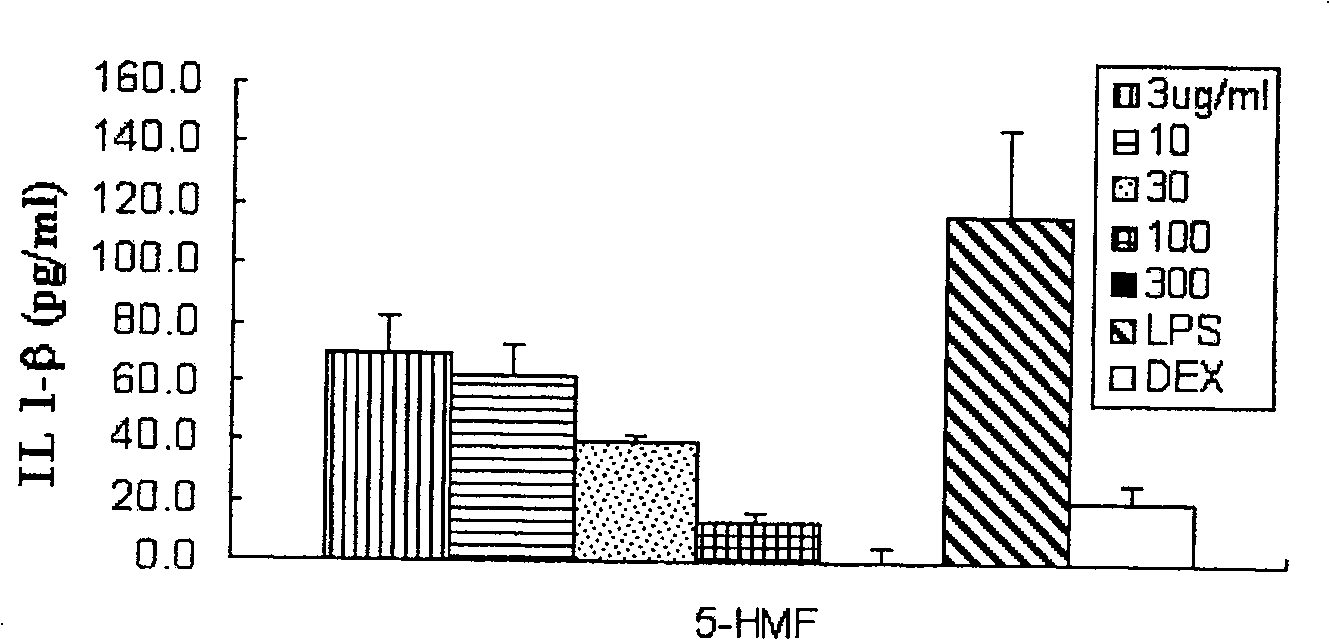5-hydroxymethyl -2-furol and its derivative analogue medical use
A kind of use, methyl technology, applied in the field of medical use of 5-hydroxymethyl-2-furfural and its derivatives, analogues, can solve undisclosed problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Separation and identification of 5-hydroxymethyl-2-furfural (5-HMF)
[0071] 500 g of Cornus officinalis was extracted 3 times with slightly boiling 0.36% hydrochloric acid aqueous solution (5000 mL), 2 hours each time. The three extracts were combined and filtered. The filtrate was passed through a HP-20 macroporous resin adsorption column (6.5×100 mm), and then eluted with water, 10% ethanol, and 15% ethanol in sequence. The 15% ethanol eluate was concentrated, then purified by RP-18 column, and water was used as eluent. After the eluate was concentrated, it was purified with a Gilson semi-preparative HPLC instrument [Zorbax C18 column (9.4×250mm)], and the mobile phase was CH 3 OH / H 2 O(8 / 92), the flow rate was 8 mL / min, and the fraction with a retention time of 5 minutes was collected and dried to obtain 100 mg of compound 5-hydroxymethyl-2 furfural (yield 0.02%).
[0072] UV(λ max , nm): 284, 230, 195;
[0073] IR (cm -1 , KBr): 3350, 1673, 1522, 1...
Embodiment 2
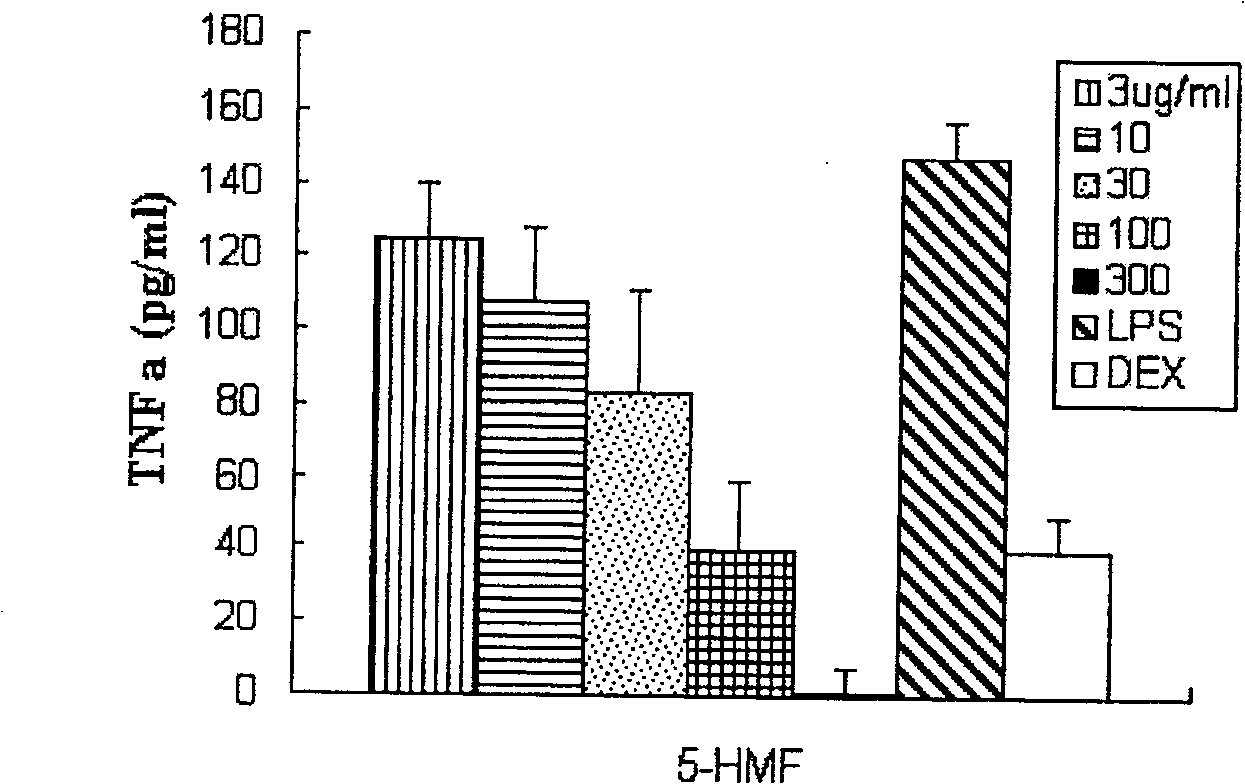
[0077] Example 2 5-Hydroxymethyl-2-furfural inhibits the expression of inflammatory factors in vitro
[0078] Experimental Materials:
[0079] 1. Cells: peripheral blood mononuclear cells
[0080] 2. Test drug: 5-hydroxymethyl-2-furfural (5-HMF)
[0081] 3. Positive control: dexamethasone
[0082] 4. Reagents: Ficoll-Paque Plus (Amersham Bioscience); endotoxin (LPS, lipopolysaccharide) and dexamethasone (CalBiochem.); TNFa ELISA Kit and IL-1βELISA Kit (Jingmei Bioengineering Company); DMSO (Sigma).
[0083] Method and Results:
[0084] Fresh blood was treated with EDTA as an anticoagulant, and peripheral blood mononuclear cells were separated and suspended in RIMP 1640 medium containing 10% FBS. Add 100 μl to a density of 1 x 10 in a 96-well plate 5 Cells / ml of freshly isolated cells, the total number of cells per well is 10 4 3 holes for each sample.
[0085] 1) Add specified concentrations of 5-hydroxymethyl-2-furfural (final concentrations are 3, 10, 30, 100, 300ug / m...
Embodiment 3
[0095] Embodiment 3 Preparation of (embodiment 1) tablet containing 5-hydroxymethyl-2-furfural
[0096] 5-Hydroxymethyl-2-furfural 30g
[0097] Starch 3g
[0098] Appropriate amount of starch slurry (10%)
[0099] Citric acid 0.15g
[0101] Mix 5-hydroxymethyl-2-furfural with starch evenly, add 8% starch slurry to make software, granulate with 14-mesh nylon sieve, dry at 70-80°C, granulate through 10-12 mesh wire sieve, and mix with talc After the powder is mixed, it is pressed into tablets with a 12mm die.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com