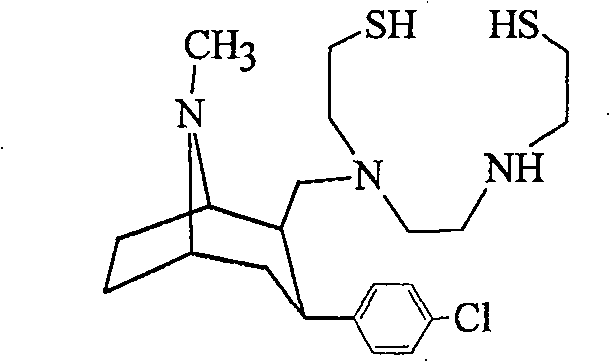
Method for preparing adamantane disulfide dinitride derivative
A technology of dimethyladamantaneaminoacetamide and dimethyladamantaneaminoacetamide, which is applied in the field of synthesis of radiopharmaceutical-labeled precursor compounds, and can solve problems such as no relevant reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
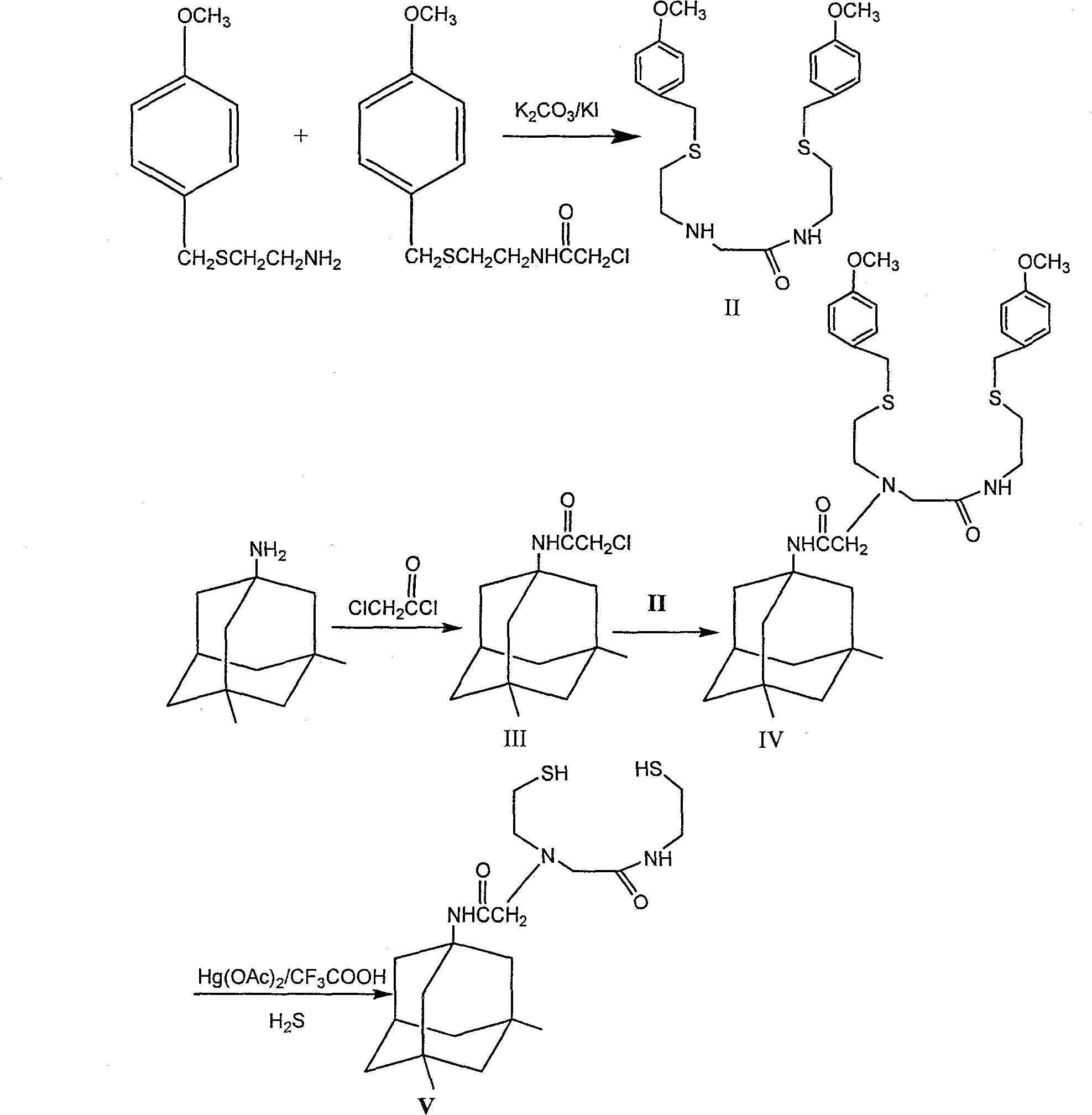
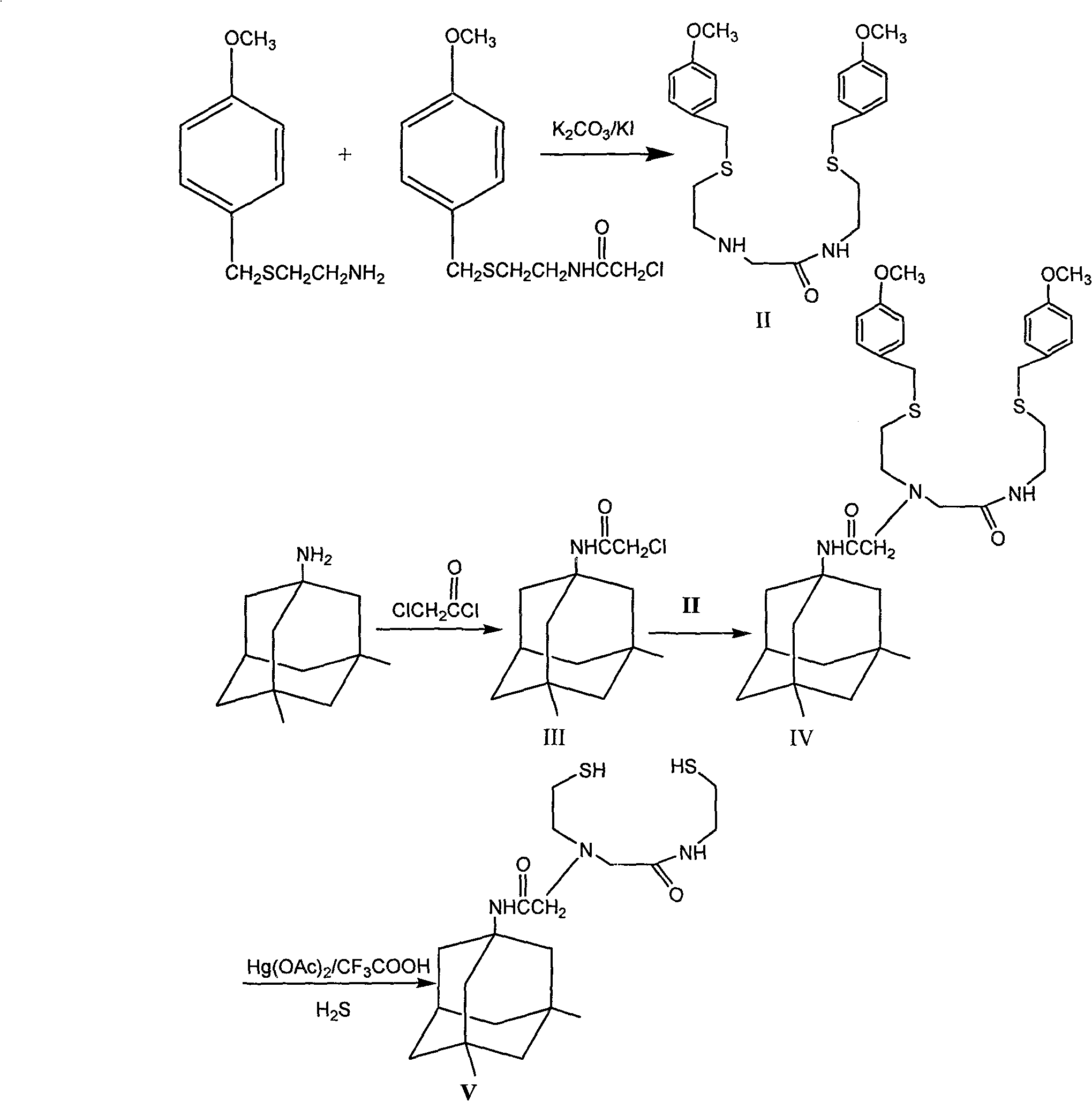
[0024] Example 1N-[2-(2-(S-(4-methoxybenzyl) mercapto) ethyl) amino]-(2-(S-(4-methoxybenzyl) mercapto) ethyl) Synthesis of Acetamide: Synthesis of (Compound II):
[0025] 4-Methoxybenzyl-(2-chloroacetamidoethyl)sulfide (5.47g, 20mmol), 2-(4-methoxybenzylthio)ethylamine (3.94g, 20mmol) and Potassium carbonate (20mmol), dry triethylamine 4.2mL, anhydrous acetonitrile 100mL, potassium iodide (15mmol), reflux for 22 hours, and the reaction was complete as detected by thin layer chromatography. Acetonitrile was removed by rotary evaporation, water and dichloromethane in equal volume ratio were added to separate layers, the organic phase was washed with water, dried over anhydrous sodium sulfate, and rotary evaporated to dryness to obtain 6.93 g of crude white solid, which was purified by column chromatography to obtain compound II as a white solid.
Embodiment 2
[0026] Embodiment 2 Bromoacetyl-3, the synthesis of 5-dimethylamantadine (compound III):
[0027] N 2 Protection, control the temperature at -45°C, dissolve (10.54g, 58.9mmol) amantadine in 75mL of dry dichloromethane, add dropwise to the solution of chloroacetyl chloride (58.7mmol) in 75mL of dichloromethane, dropwise, dropwise Triethylamine 10mL, continue to stir for 1 hour, rise to room temperature and stir for 1 hour, add 150mL of water to separate layers, wash the organic layer with water until neutral, dry with anhydrous sodium sulfate, and rotary evaporate to dryness to obtain 13.4g of crude light yellow solid. Ethyl ester:n-hexane (1:6) was recrystallized to obtain pale yellow granular crystals (compound III).
Embodiment 3
[0028] Example 3N-[2-(S-(4-methoxybenzyl)thio)ethyl]N-[(N-(2-(S-(4-methoxybenzyl)thio)ethyl Base) amino) formylmethyl] -3, the synthesis of 5-dimethyladamantanylaminoacetamide (compound IV):
[0029]Compound II (1.6g, 3.6mmol), compound III (0.98g, 3.6mmol), potassium carbonate (5g, 36mmol), potassium iodide (0.6g, 3.6mmol), anhydrous acetonitrile 100mL, reflux reaction for 12 hours, thin layer Chromatographic detection of complete reaction, spin-dried anhydrous acetonitrile, added 100mL water and 100mL dichloromethane for layering, washed the organic layer with water until neutral, dried over anhydrous sodium sulfate, and spin-dried to obtain 2.2 g of a dark red oily crude product, dissolved in ethyl acetate : n-Hexane (1:5) as the eluent column chromatography to obtain translucent oil (compound IV).
[0030] HPLC: 97%.
[0031] Mass Spectrum MS: MS (m / z): 654(m+1), 676(m+Na), 692(m+K), 708(m+NaS) 2 methyl groups on 1H-NMR adamantane -0.832ppm (6H singlet), 1.10-1.18ppm (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com