A lonicera and scutellaria drip pill and its preparation method
A technology of yellow dripping pills and dripping pills, which is applied in the field of medicines, Yinhuang dripping pills and their preparation, can solve the problems of long disintegration time limit, slowing down the release speed of active ingredients, and poor dissolution in vitro, so as to facilitate dissolution and fully The effects of absorption, flexible and accurate dosage, and stable drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: prepare Yinhuang dripping pills
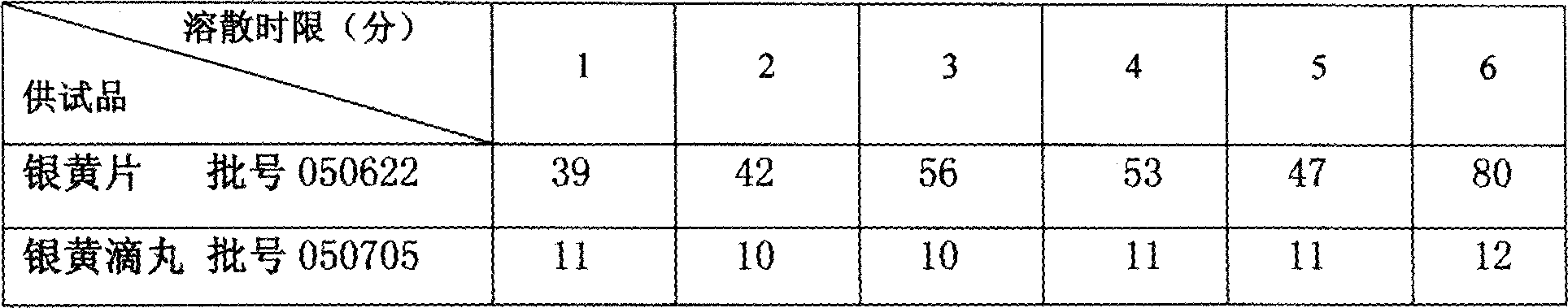
[0038] by WS 3 -B-1233-92 Prepare 10g of honeysuckle extract and 4g of Scutellaria baicalensis extract, crush them through a 100-mesh sieve, and set aside, take 46g of polyethylene glycol 4000, heat and melt in a water bath at 80°C, add honeysuckle extract and Scutellaria baicalensis extract under stirring Stir at 80°C for 1 hour to disperse evenly, and transfer the liquid medicine to the 80°C constant temperature storage tank of the dropping pill machine. Methyl silicone oil, under the condition of cooling liquid temperature 20 ± 3 ℃, adjust the weight of dropping pills to 60mg, drop into pills, remove the cooling liquid on the surface of dropping pills, pack separately, and obtain 14 mg of silvery yellow extract (honeysuckle extract 10mg: Scutellaria baicalensis extract 4mg) and polyethylene glycol 4000 46mg Yinhuang drop pills.
experiment example 1
[0040] Experimental Example 1: The applicant explored and screened out a reasonable process for preparing Yinhuang dripping pills through a large number of experiments.
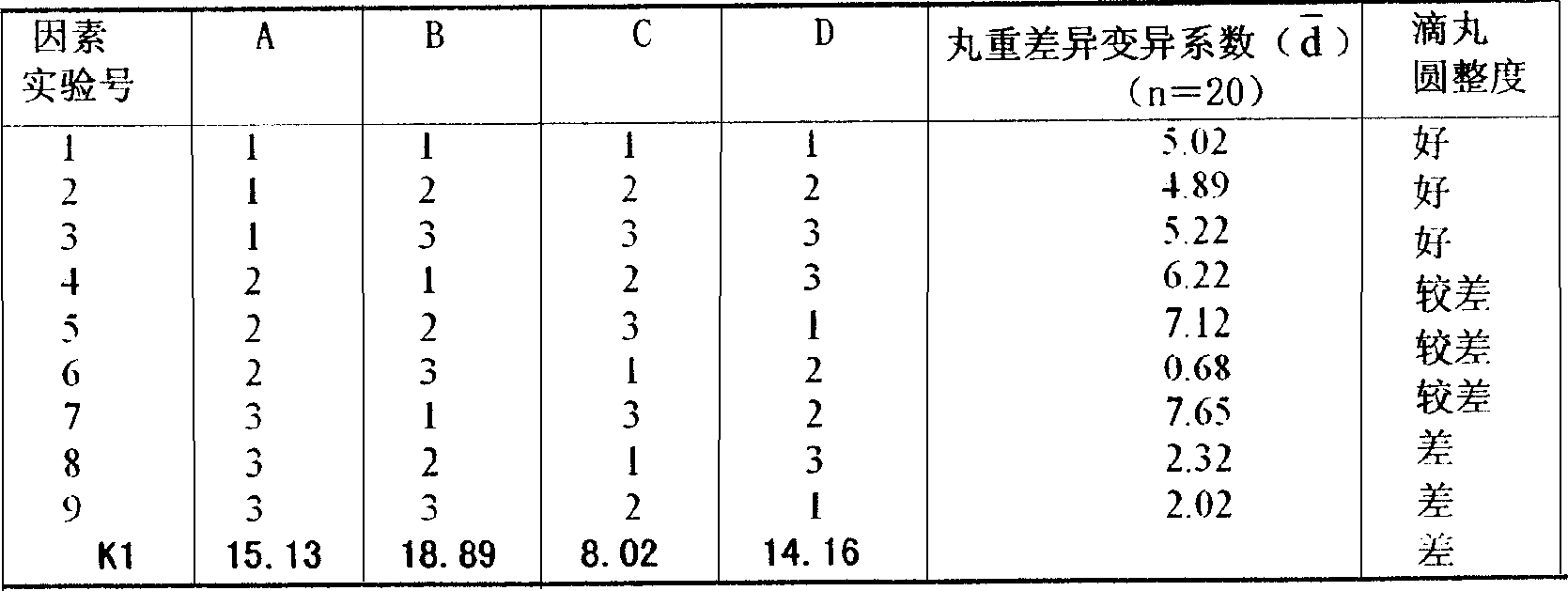
[0041] (1) Excipient screening:
[0042] Dropping pill bases include water-soluble bases and water-insoluble bases, commonly used polyethylene glycols (such as polyethylene glycol 6000, polyethylene glycol 4000, etc.), poloxamer, polyoxyl stearate (40 ) ester (S-40), gelatin, stearic acid, glyceryl monostearate, hydrogenated vegetable oil, etc. The condensate of dripping pills must be safe and harmless, and have no effect on the main drug. The commonly used ones are liquid paraffin, vegetable oil, methyl silicone oil and water. Polyethylene glycol is a commonly used solid dispersion matrix for dropping pills. It has stable properties and is not easy to chemically react with drugs. Therefore, we prefer polyethylene glycol as the dispersion matrix. On the basis of the maximum drug loading, the fluidity of the...
experiment example 2
[0055] Experimental Example 2: Recipe Optimization
[0056] From the above orthogonal test results, it can be seen that the current optimal prescription process is not perfect, and the fluidity of the liquid medicine is not very good. In order to make the preparation process prescription of this product more reasonable, the preparation process is more suitable for industrial production, so on the basis of the above orthogonal test results On the basis of further optimization of the current best process prescription, the fluidity of the liquid medicine can be improved by adding an appropriate amount of regulators. The investigation methods are shown in Table 4.
[0057] Inspection method:
[0058] With the temperature of the liquid medicine at 80°C and simethicone oil as the coolant, prepare the liquid medicine according to the prescription in Table 4, stir for 40 minutes under heat preservation, and let stand for 2 hours under heat preservation to investigate the fluidity and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com