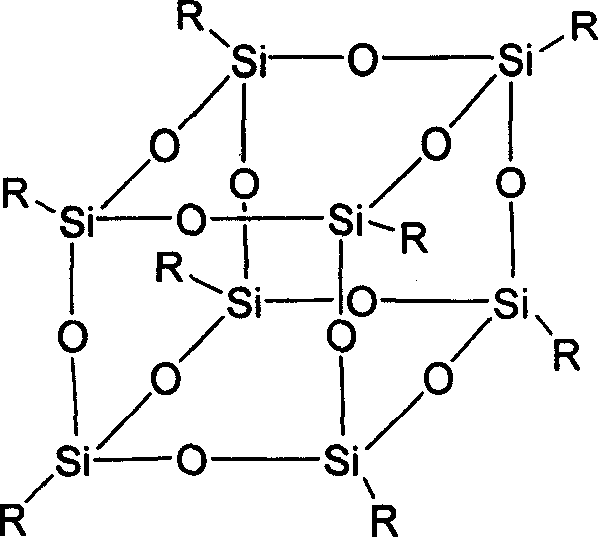
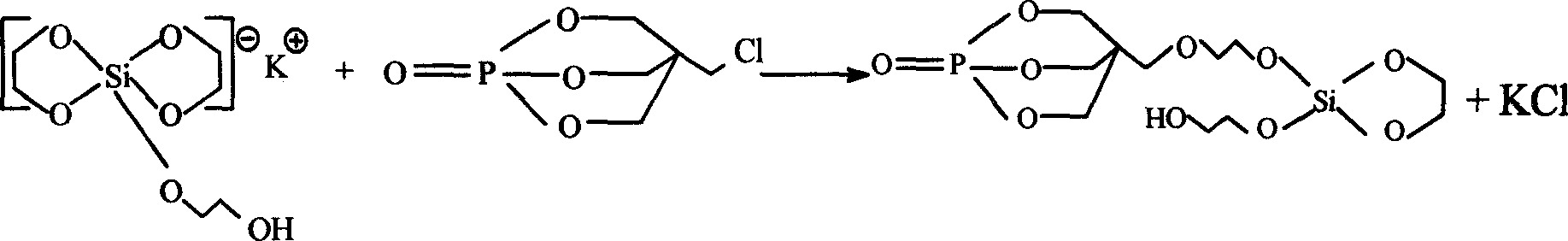
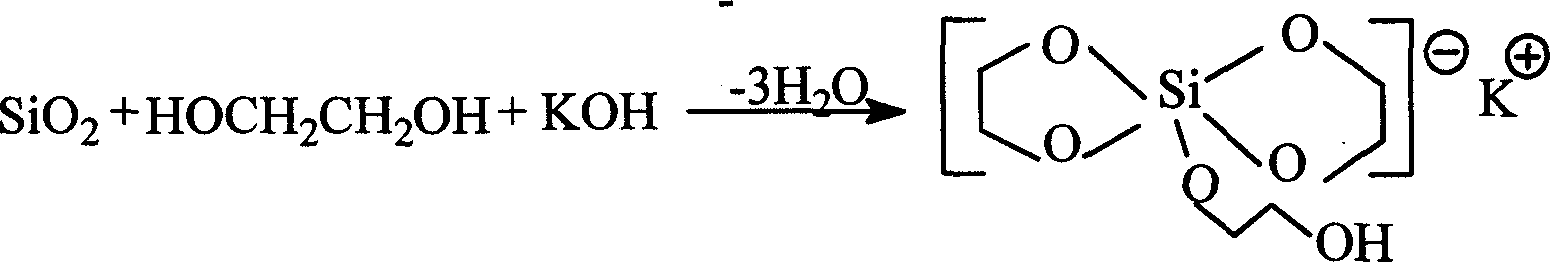Double-ring cage shape substituted silsesquioxane and its preparation method and uses
A technology of silsesquioxane and ring cage, applied in the field of polymer additives synthesis, can solve the problems of poor impact resistance, crack resistance and humidity and heat resistance, large internal stress and high cross-linking density, and achieve good permeability. effect, the effect of improving weather resistance and abrasion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Stir and mix 36.4mL of 25% tetramethylammonium hydroxide aqueous solution with 54.6mL of distilled water, then add 1-oxo-1-phospha-2,6,7-trioxa in 3 times within 1h Bicyclo[2,2,2]octane-4-methylenealkoxy substituted tetracoordinated silicon (0.1mol), vigorously stirred during the feeding process to accelerate dissolution, stirred at 25°C for 20h, then heated to 65°C for 5h, Stop the reaction, remove a small amount of insoluble matter by suction filtration, the filtrate is light yellow, change to a distillation device, evaporate about 30mL of solvent, and place it in a refrigerator at 5°C to cool. After two days, a large amount of crystals are produced, take them out, and place them at room temperature for 1 hour to let them After the ethylene glycol crystals were dissolved, they were suction filtered, and the product was cleaned with 60 mL of acetone, and then dried at 60°C for 48 hours to obtain a white granular substance, which was a bicyclic cage-like substituted sils...
Embodiment 2
[0033] Potassium hydroxide (0.01mol) and methanol aqueous solution (79%wt, 68.8mL) were stirred and mixed evenly, and then 1-oxo-1-phospha-2,6,7-trioxa was added in portions within about 1 h Bicyclo[2,2,2]octane-4-methylenealkoxy was substituted with tetracoordinated silicon (0.1mol). During the process, vigorously stirred to accelerate the dissolution. The reaction was stirred at 30°C for 20h, and the temperature was raised to 60°C for 6h. Filter out a small amount of insoluble matter, the filtrate is light yellow, change to a distillation device, evaporate about 30mL of solvent, and then cool it in the refrigerator. After two days, a large amount of crystals are produced, take them out, and place them at room temperature for 1 hour to dissolve the ethylene glycol crystals. Afterwards, suction filtration, the product was washed with acetone (70 mL) and dried at 60° C. for 48 hours to obtain a white granular substance which was a bicyclic cage-shaped substituted silsesquioxane ...
Embodiment 3
[0036] Concentrated hydrochloric acid (2×10 -3 mol) and ethanol aqueous solution (25.4%wt, 36.2mL) were stirred and mixed evenly, and then 1-oxo-1-phospha-2,6,7-trioxabicyclo[2,2, 2] Octane-4-methylenealkoxy is substituted with tetracoordinated silicon (0.1mol). During the process, stir vigorously to accelerate the dissolution. Stir and react at room temperature (28°C) for 24h, heat up to 70°C for 5h, and remove it by suction filtration A small amount of insoluble matter, the filtrate was light yellow, changed to a distillation device, evaporated about 20mL of solvent, placed it in the refrigerator to cool, two days later a large number of crystals were produced, took it out, and placed it at room temperature for 1 hour to dissolve the ethylene glycol crystals, then pumped After filtering, the product was washed with acetone (80 mL) and dried at 60° C. for 48 hours to obtain a white granular substance which was a bicyclic cage-shaped substituted silsesquioxane according to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Rockwell hardness | aaaaa | aaaaa |
| oxygen index | aaaaa | aaaaa |
| Rockwell hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com