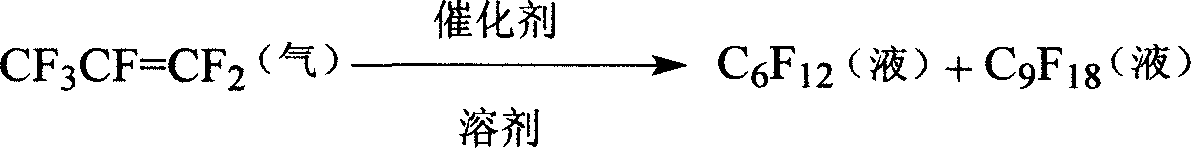
Process of preparing hexafluoropropylene oligomer
A technology of hexafluoropropylene and oligomer is applied in the field of preparation of hexafluoropropylene oligomer to achieve the effects of less three wastes, good selectivity and improved selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Measure 40mL of dimethyl sulfoxide into the autoclave, then add 2.5g of KF, 2.5g of N,N-xylaniline and 2.5g of polyethylene glycol 600 respectively, cover and seal, connect the vacuum device, remove The air in it was fed with nitrogen, and then vacuumized to make the pressure in the kettle zero, and the ventilation operation was carried out three times. Then feed hexafluoropropylene gas to make the pressure reach 0.6MPa, turn on the stirrer, 400 rpm, heat to 70°C, keep the pressure and temperature basically unchanged, and close the hexafluoropropylene inlet valve after 90 minutes. Continue to stir to normal pressure, blow nitrogen to 0.6MPa, react at 70°C for 30 minutes, stop stirring, and cool. Then the reactant was transferred to a 1L pear-shaped separatory funnel and left to stand overnight. After separation, the light yellow fluorocarbon in the lower layer was obtained, which was washed with water and dried. According to gas chromatography analysis, the mass conten...
Embodiment 2
[0029] The three-way catalyst was changed to 2.0g KF, 2.0g diethylamine and 2.0g polyethylene glycol nonylphenyl ether, and other reactions and post-treatment were the same as in Example 1. The mass content of perfluorohexene in the hexafluoropropylene oligomerization product is 1.4%, the mass content of perfluorononene is 93.2%, and the mass content of perfluorononene after rectification treatment is 99.5%.
Embodiment 3
[0031] The three-way catalyst was changed to 1.5g KF, 2.0g triethylamine and 2.5g polyethylene glycol nonylphenyl ether, and the reaction temperature was changed to 150°C. Other reaction conditions and post-treatment were the same as in Example 1. The mass content of perfluorohexene in the hexafluoropropylene oligomerization product is 1.1%, the mass content of perfluorononene is 93.2%, and the mass content of perfluorononene after rectification treatment is 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com