Method for synthesizing polysilane containing two bonds
A synthesis method and technology of polysilane, applied in the field of synthesis of polysilane, can solve the problems of inability to form double bond-containing polysilane, unfavorable large-scale production of polysilane, low retention rate of polysilane double bonds, etc. It is beneficial to large-scale industrial application and the effect of reducing mechanical damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: In an electrochemical reaction flask equipped with a ventilation tube, a stirrer and a thermometer, methyltrichlorosilane and allyl chloride in different molar ratios were respectively added. Then add about 130ml of tetrahydrofuran (add a small amount of sodium for dehydration and distill, take a fraction at 66°C) and lithium perchlorate (LiClO) with a concentration of 0.1mol / L 4 ) as the supporting electrolyte. Magnesium blocks are used as cathode and anode. After three times of vacuuming and nitrogen filling, start stirring and ultrasonic wave, and then turn on the electricity to start the reaction. When the reaction power reaches 1340mA*h, stop the electricity, add 200ml of toluene, and pass in a balloon of ammonia gas to neutralize the remaining Si-Cl bonds. After secondary pressure filtration and vacuum distillation, a light yellow liquid polysilane containing double bonds is obtained. The molar ratio of methyltrichlorosilane and allyl chloride used...
Embodiment 2
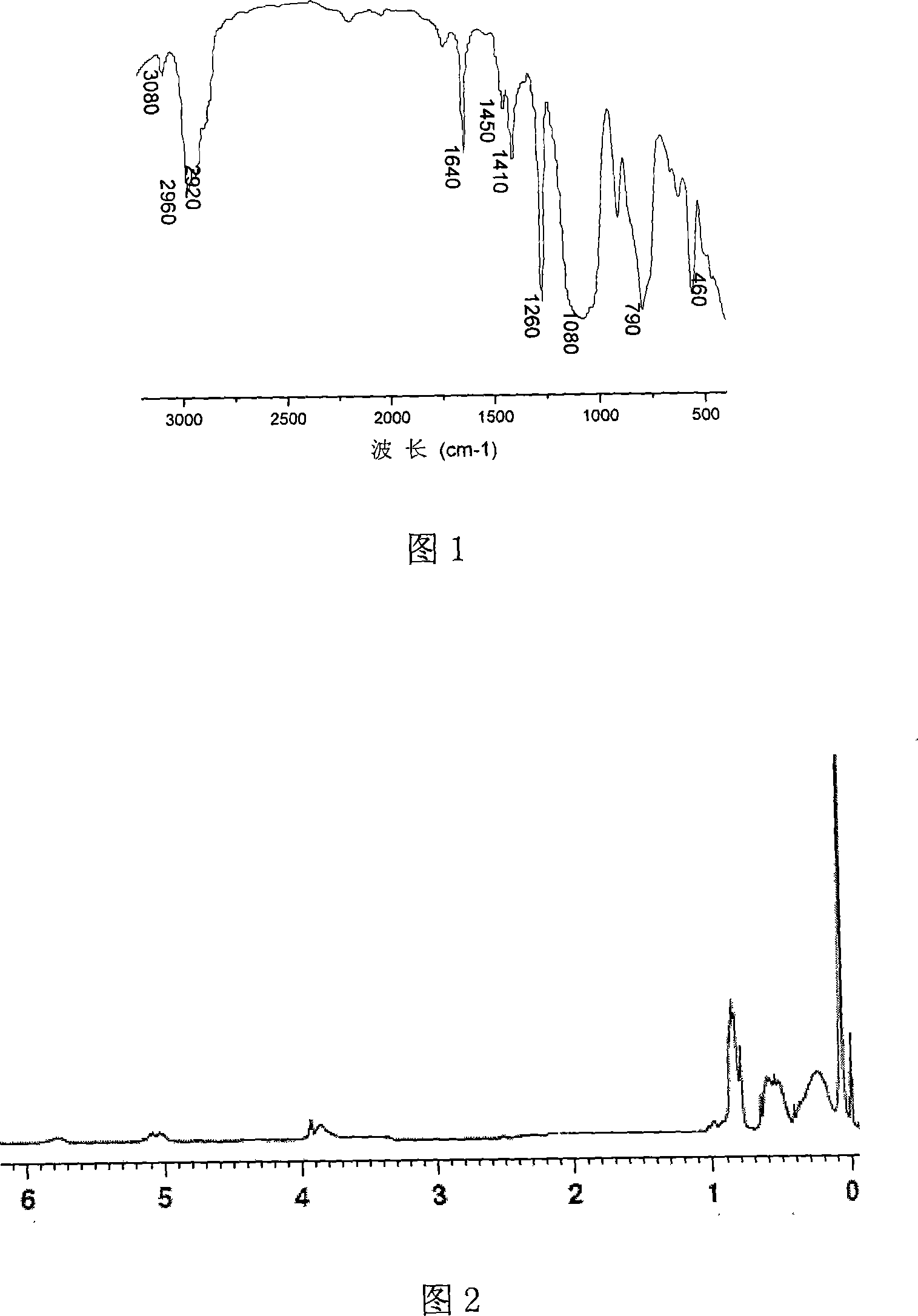
[0023] Example 2: The infrared spectrogram of polysilane containing double bonds was prepared according to the addition ratio of methyltrichlorosilane and allyl chloride molar ratio of 3 / 1, as shown in FIG. 1 . Among them 2960, 2920cm -1 The peak corresponds to the polysilane CH 3 - Stretching vibration of upper saturated C-H; 1450, 1410cm -1 for Si-CH 3 Deformation vibration of middle C-H; 1260cm -1 for Si-CH 3 Middle CH 3 Deformation vibration; 1080cm -1 It is the characteristic absorption peak of the silicon-oxygen bond, which is caused by the inability to completely isolate oxygen during the experiment; 790, 690cm -1 for Si-CH 3 stretching vibration; 460cm -1 It is the characteristic absorption peak of silicon-silicon bond. This indicates that polysilanes containing double bonds have Si-CH 3 , saturated C-H, Si-Si and other structural groups. At the same time, polysilanes containing double bonds also have some special double bond absorption peaks: 1640cm -1 It ...
Embodiment 3
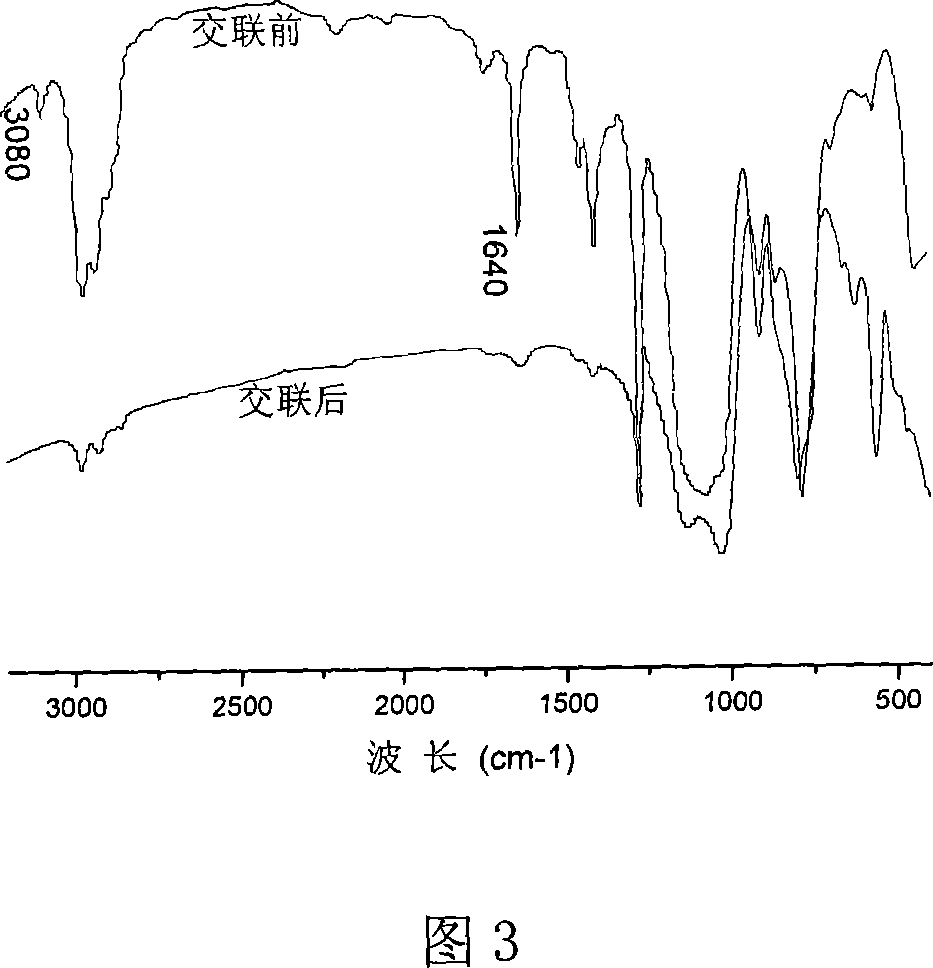
[0024] Embodiment three: prepare the polysilane containing double bond by the addition ratio of methyltrichlorosilane and allyl chloride molar ratio 3 / 1 1 HNMR spectrum, as shown in Figure 2. Among them, the peaks with chemical shift δ at 0.09, 0.25, 0.5-0.6 and 0.85 belong to polysilane Si-CH 3 Structural unit, because Si-CH in polysilane containing double bond 3 The structure around the group is very complex, so in its 1 Si-CH in HNMR spectrum 3 The peak shape is broad and complex. The spectral peaks with chemical shift δ at 5.8, 5.1-5.2, and 3.8-3.9 are assigned to the H on the one, two, and three-position carbons of the allyl group, respectively. This further proves that the synthesized polysilane has an allyl structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com