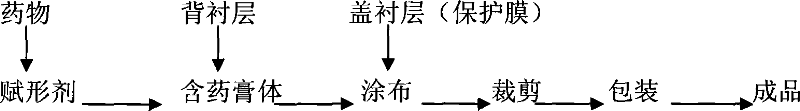
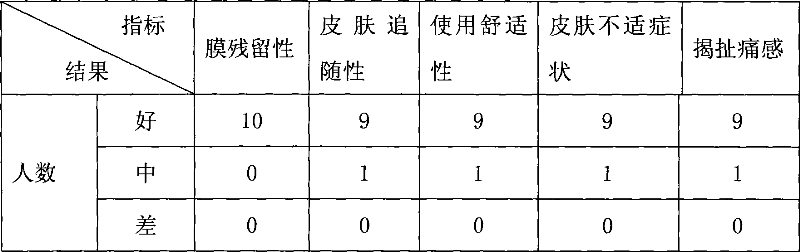
Compound panax notoginseng cataplasma
A technology of compound panax notoginseng and babu agent, applied in the directions of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of prone to allergic reactions, gastrointestinal discomfort of patients, poor patient compliance, etc., to avoid Liver first-pass effect, favorable for transdermal absorption, and long-lasting effect of drug action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Backing layer: non-woven fabric
[0062] Cover lining: polyester film
[0063] The composition of the medicated ointment layer is as follows by weight:
[0064] Compound Panax notoginseng extract (micronized dry extract powder) 1.5g
[0065] Gelatin 1.5g
[0066] Tragacanth Gum 1.5g
[0067] Macrogol-400 1.3g
[0068] Polyacrylic acid 1.0 g
[0069] Glycerin 0.5g
[0071] Azone 0.2g
[0072] borneol 0.05g
[0073] water 7 grams
[0074] Preparation method: crush the extract of compound notoginseng, weigh gelatin and tragacanth gum according to the prescription, add water, heat in a water bath to dissolve, mix polyacrylic acid, glycerin and zinc oxide evenly, add to the above glue, stir and mix evenly , then add polyethylene glycol-400 to disperse the suspension of borneol and compound notoginseng extract, and finally add azone to obtain a paste. Then spread it evenly on the non-woven fabric, cover it with a polyester film, and cut i...
Embodiment 2
[0077] Backing layer: non-woven fabric
[0078] Cover lining: aluminum foil-polyethylene composite film
[0079] The composition of the medicated ointment layer is as follows by weight:
[0080] Compound Panax notoginseng extract (dry extract powder) 1.0g
[0081] Sodium Polyacrylate 2.0g
[0082] Gelatin 1.5g
[0083] 1.5 g polyvinyl alcohol
[0084] Glycerin 2.0g
[0086] Azone 0.3g
[0087] Propylene Glycol 1.0g
[0088] Menthol 0.06g
[0089] water 17.0g
[0090] Preparation method: crush the extract of compound notoginseng, weigh gelatin, sodium polyacrylate, and polyvinyl alcohol according to the prescription, add water to make swelling, stir and mix evenly, mix glycerin, zinc oxide, and azone evenly, add the above swelling In the liquid, stir and mix evenly, then add the suspension liquid in which the menthol and compound Sanqi extract are dispersed with propylene glycol and azone to obtain a paste. Then spread it evenly on the non-wov...
Embodiment 3
[0093] Backing layer: non-woven fabric
[0094] Lid lining: Polyethylene film
[0095] The composition of the medicated ointment layer is as follows by weight:
[0096] Compound Panax notoginseng extract (micronized dry extract powder) 0.8g
[0097] Sodium polyacrylate 1.8g
[0098] Carbomer 934p 0.3g
[0099] Glycerin 5.3g
[0100] Propylene Glycol 1.4g
[0101] Kaolin 1.8g
[0102] Citric acid 0.04g
[0103] Aluminum trichloride 0.08 g
[0104] Menthol 0.07g
[0105] Purified water 25.0g
[0106] Preparation method: 1) Weigh sodium polyacrylate and carbomer according to the prescription amount, add water to make swelling, add aluminum trichloride, stir and mix evenly; 2) Mix glycerin and kaolin and add to 1; 3) Use propylene glycol to dissolve mint Brain and compound Panax notoginseng extract powder were dissolved into a suspension and added to 2; 4) Finally, citric acid was added, stirred and mixed evenly to obtain a paste. Then spread it evenly on the non-woven ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com