Multifunctional ion liquid system, preparation and application thereof
A technology of ionic liquid and composite function, which is applied in the field of composite functional ionic liquid system and its preparation, can solve the problems of poor solubility, limited product use, high price, etc., and achieve activity and stability promotion, good thermal stability, super The effect of low vapor pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
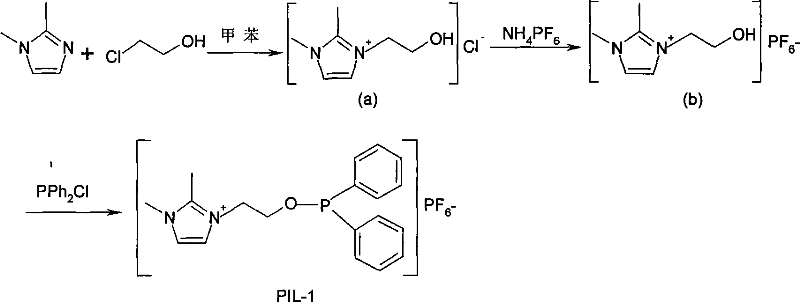
[0035] Example 1: Synthesis of PIL-1 (O-(1-ethyl-2,3-dimethylimidazole)-diphenylphosphinite hexafluorophosphonate):
[0036]
[0037] (a) Preparation: Under the protection of nitrogen, add 48 g (0.5 mole) of 1,2-dimethylimidazole to a 250 ml three-necked flask, add 40 ml of toluene and 50 ml of chloroethanol (0.75 mole), and set the temperature The temperature was raised to 90°C and reacted for 12 hours. A large amount of oily liquid was formed in the lower layer. The reaction solution was cooled to room temperature, and a molecular sieve was added as a crystal nucleus. After being placed in a refrigerator for 24 hours, there were a large amount of white crystals. After washing with acetone several times, suction filtration, oil pump drying, a white solid (a) was obtained. The solid is hygroscopic, soluble in ethanol, but insoluble in acetone and ether.
[0038] (b) Preparation: In 19 g (a) (0.13 mol) of the aqueous solution, 21 g NH dissolved in it was added by filtration 4 PF ...
Embodiment 2-3
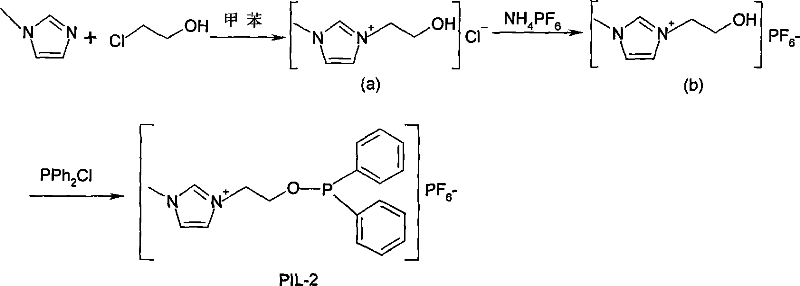
[0040] Example 2-3: Ionic liquid PIL-2 (O-(1-ethyl-3-methylimidazole)-diphenylphosphinate hexafluorophosphonate) grafted with phosphine ligand and PIL-3 The synthesis of (O-(1-(2)-phenyl-ethyl)-3-methylimidazole)-diphenyl hypophosphite hexafluorophosphonate):
[0041]
[0042] The synthesis method and test procedure of PIL-2 are the same as PIL-1. The starting materials were changed to 1-methylimidazole and 2-chloroethanol.
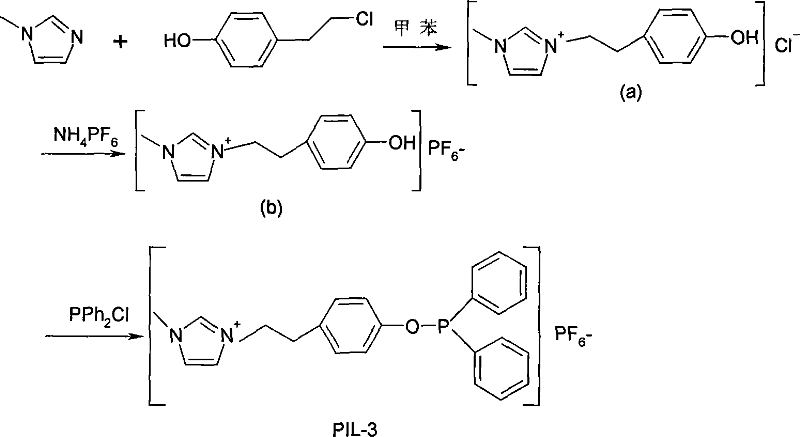
[0043]
[0044] The synthesis method and test procedure of PIL-3 are the same as PIL-1. The starting materials were changed to 1-methylimidazole and 4-(2-chloroethyl)phenol.
Embodiment 4
[0045] Example 4: Synthesis of PIL-4 (1-ethyl-2,3-dimethylimidazole-diphenylphosphine hexafluorophosphonate):
[0046]
[0047] (a) Preparation: N 2 Dissolve 45g (0.47mol) of 1,2-dimethylimidazole in 50ml of toluene under protection, then add 32ml of 1,2-dichloroethane (0.41mol), stir at 80℃ for 24 hours, then cool After standing at room temperature overnight, a milky white solid precipitated out, the supernatant liquid was discarded, and the white solid was washed with toluene and acetone respectively.
[0048] (b) Preparation: 29 g (a) (0.15 mol) is placed in a 250 ml single-necked bottle, dissolved with as little water as possible, and 29 g of ammonium hexafluorophosphate (NH 4 PF 6 ) Aqueous solution, stirred for half an hour, suction filtered, washed with water and ethanol several times, and finally dried under vacuum to obtain (b).
[0049] Preparation of PIL-4: Under the protection of nitrogen, the prepared lithium diphenylphosphine (PPh 2 Slowly add 6 g (b) (0.031 mol) t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com