Esterified object of maleic anhydride of styrene, preparation method, and application
A technology of styrene maleic acid and maleic anhydride, which can be used in drug delivery, pharmaceutical formulation and other directions, and can solve the problems of long reaction time, low esterification rate, and many by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] Examples (in list form)
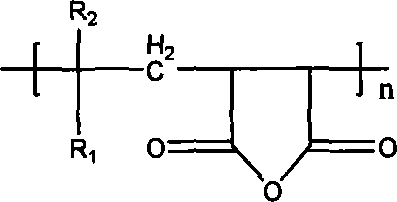
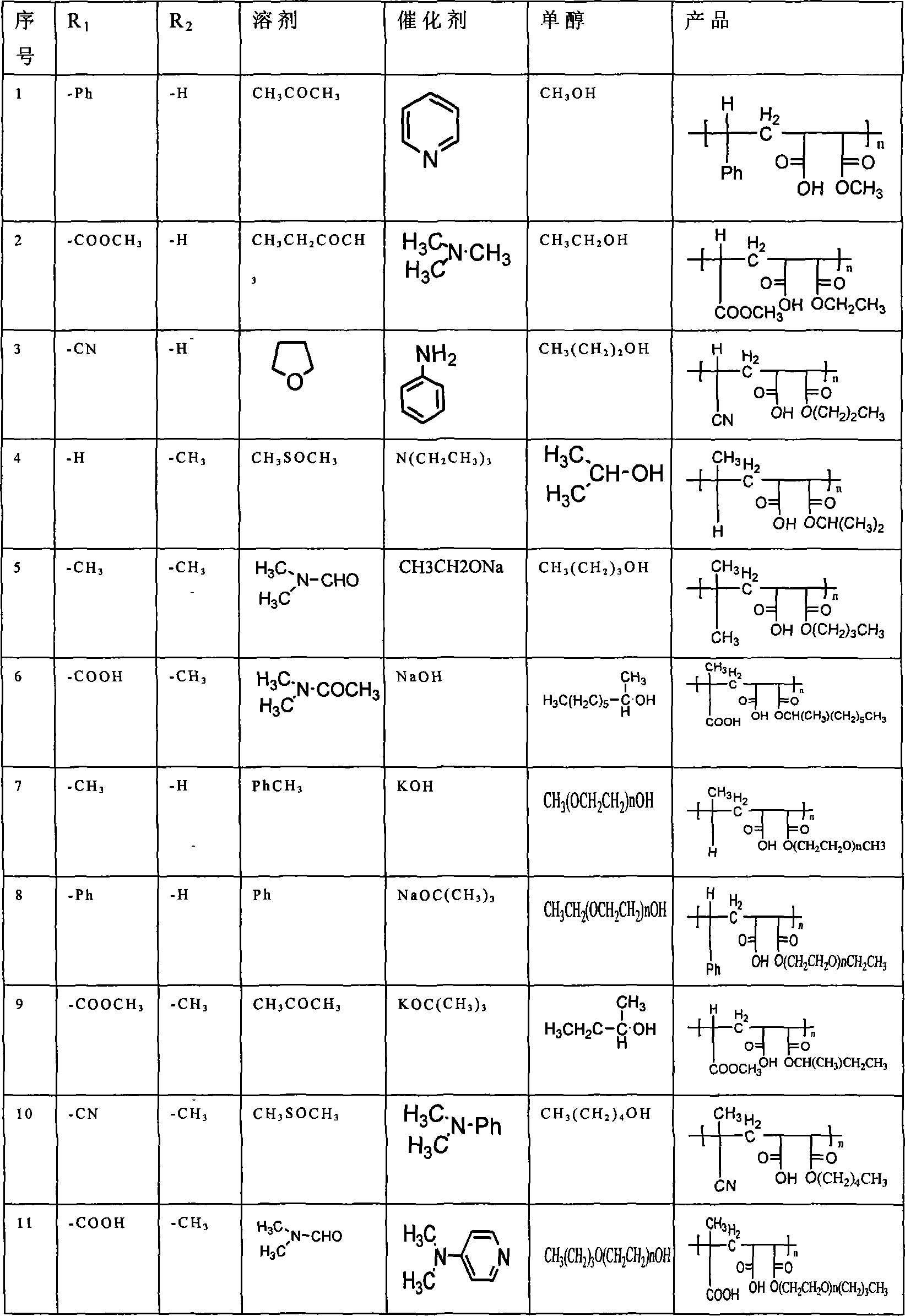
[0018] a. First select two reactants, one with The structure of reactants, where R 1 is phenyl or methyl carboxylate or cyano or hydrogen or methyl or carboxyl, R 2 is hydrogen or methyl; another monoalcohol reactant is methanol or ethanol or n-propanol or isopropanol or n-pentanol or isobutanol or n-pentanol or ethyl octanol or polyethylene glycol monomethyl ether or Polyethylene glycol monoethyl ether or polyethylene glycol monobutyl ether;
[0019] B, these two kinds of reactants are placed in solvent acetone or butanone or tetrahydrofuran or dimethyl sulfoxide or N, N-dimethylformamide or N, N-dimethylacetamide or toluene or benzene to react, then The mass ratio of the reactants is 0.001%-30% of the catalyst pyridine or trimethylamine or triethylamine or aniline or N,N dimethylaniline or ethylenediamine or 4-dimethylaminopyridine or sodium ethoxide or sodium hydroxide Or potassium hydroxide or sodium tert-butoxide or potassium tert-but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com