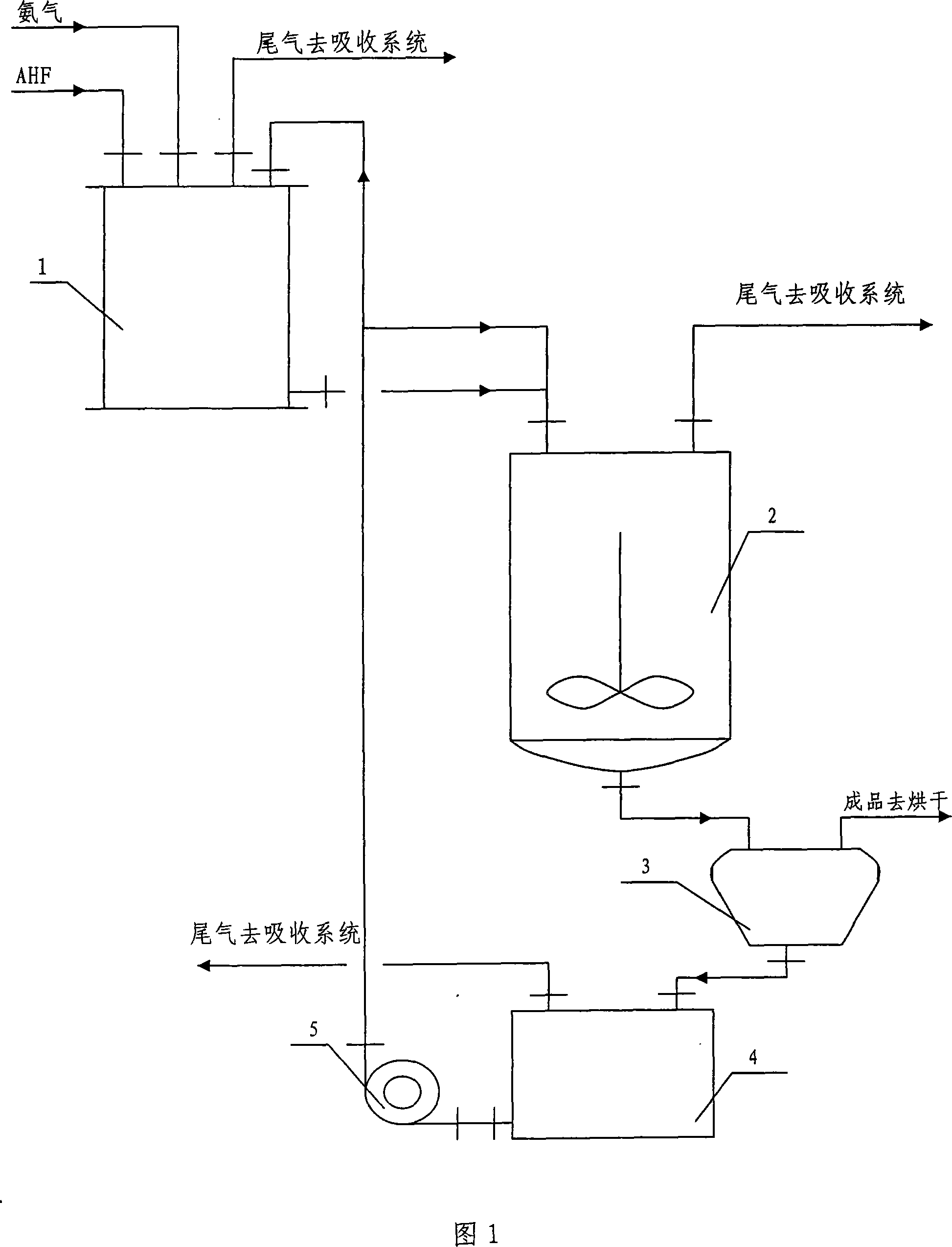
Improved wet process and apparatus for producing ammonium bifluoride
A technology of ammonium bifluoride and process, which is applied in the field of wet production ammonium bifluoride process and equipment improvement, can solve the problems of uneven crystal particle size, unrecovered tail gas, poor heat transfer effect, etc., and achieve good product quality and use Long life and less pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] According to the above process flow and steps, feed 900Kg AHF, 390KgNH into reactor 1 3 , the reaction temperature is 95°C, and when the pH value at the end of the reaction is 3.2, the temperature of the mother liquor in the crystallization tank 2 is 80°C, and the cooling rate is 1-2°C / h. The ammonium bifluoride product output, quality and consumption situation that obtain are shown in Table 1.
[0031] Output T
Embodiment 2
[0033] According to the operation procedure of embodiment 1, the pH value of the reaction end point is 2, 3, 4, 5 four conditions, when other conditions are constant, the ammonium bifluoride product yield, quality and consumption situation are shown in Table 2.
[0034] PH value
[0035] Conclusion: Other conditions remain the same, when the pH control value of the reaction end point is changed, the appearance of the product remains unchanged; with the increase of the pH control value of the reaction end point, the output decreases, the main content decreases, the acid consumption of AHF decreases, and the NH 3 Consumption increases; therefore, the pH value at the end of the reaction is preferably controlled at 3-4.
Embodiment 3
[0037] According to the operation steps of Example 1, the crystallization cooling rate is controlled under four conditions of 0~1, 1~2, 2~3, 3~4°C / h, and when other conditions are constant, the output, quality and consumption of the ammonium bifluoride product obtained See Table 3.
[0038] Cooling rate °C / h
[0039] Conclusion: Changing the cooling rate of crystallization has no effect on the output, main content, and consumption of raw materials of the product; but with the acceleration of the cooling rate, the product has uneven fineness, easy agglomeration, and different shapes, and the crystallization kettle is easy to form walls. Therefore, the cooling rate is best controlled at 0-2°C / h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com