Method for inducing stem cells to differentiate to blood vessel smooth muscle cells
A technology for vascular smooth muscle and stem cell differentiation, which is applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., and can solve the problems of easy aging and poor in vitro proliferation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Method for inducing and differentiating bone marrow mesenchymal stem cells into vascular smooth muscle cells
[0052] (1) Extract 5ml of bone marrow from the anterior superior iliac spine of healthy adults, transfer it into a 5ml sterile centrifuge tube containing the same amount of DMEM-LG culture medium (containing 10% FBS, heparin 100U / ml), centrifuge at 300g for 5 minutes, and suck off the upper layer Fat and part of the supernatant;
[0053] (2) Add on 1.073g / ml Percoll separation solution (purchased from Pharmacia, Sweden) at 1:2, and centrifuge at 900g for 30 minutes;
[0054] (3) Take the cloudy mononuclear cell layer in the middle layer, rinse with PBS, centrifuge at 300g for 5min, and discard the supernatant;
[0055] (4) Cells were collected, resuspended in low-glucose essential medium (DMEM-LG, purchased from Gibco, USA), and mixed with 2×10 5 / cm 2 Density inoculation culture, first change the medium after 48 hours of culture, wash once with PBS, remove ...
Embodiment 2
[0058] Detect the cells obtained in Example 1
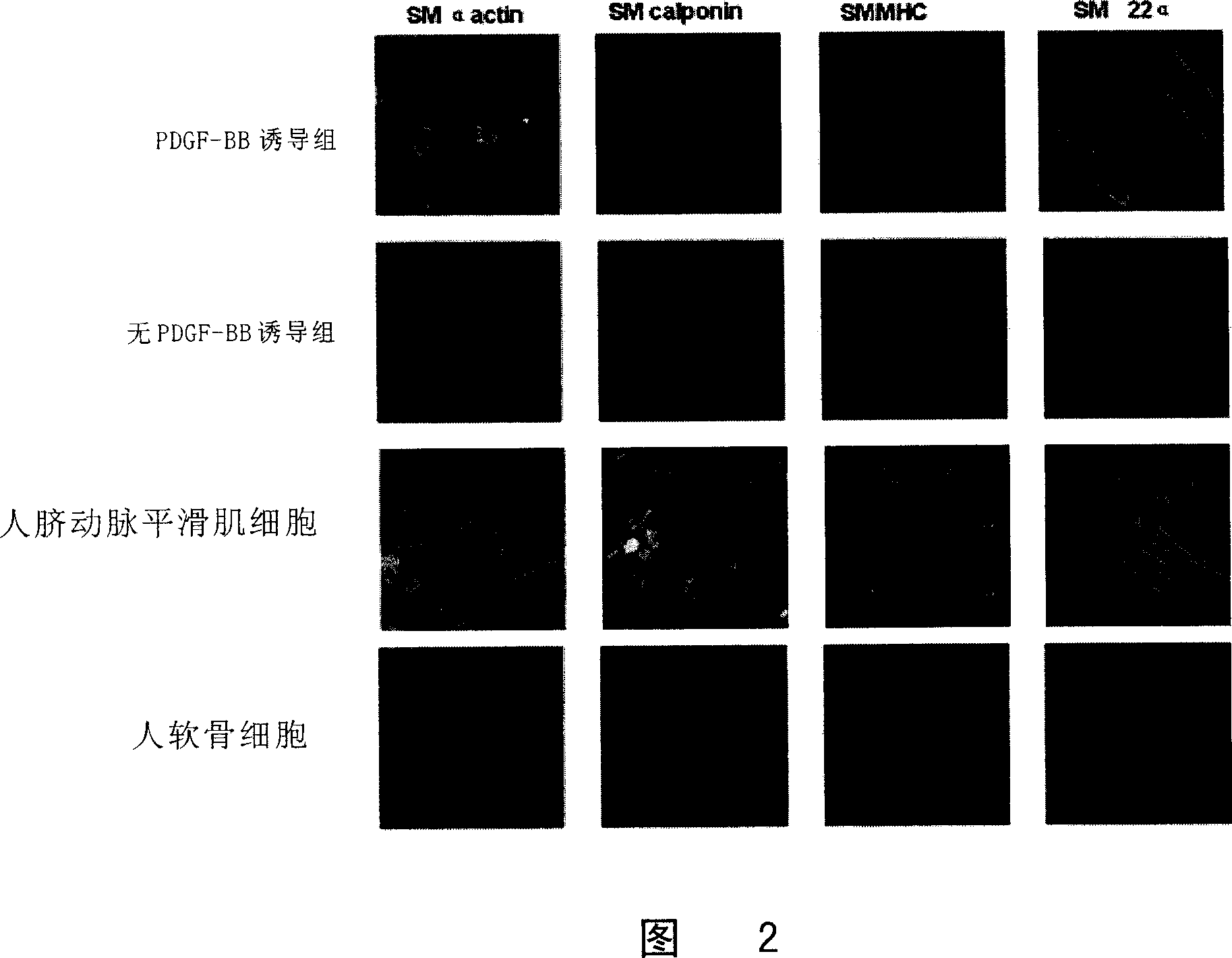
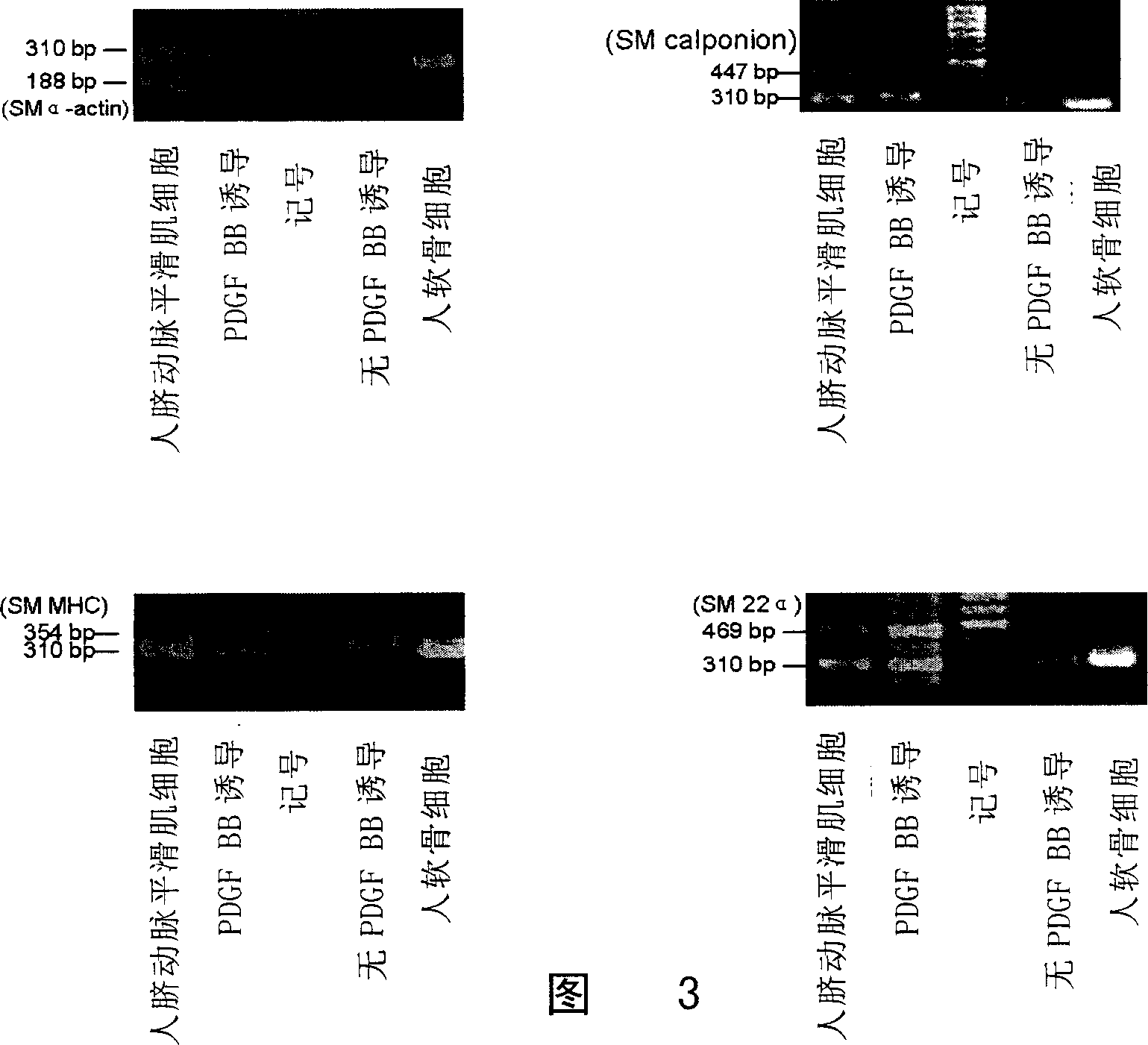
[0059] 1. Immunofluorescent staining of cells:
[0060]Cells were subcultured for 14 days, fixed with ethanol:acetic acid (99:1) for 20 minutes, rinsed with PBS for 5 minutes x 3 times; added 0.25% Triton X-100 for 10 minutes, rinsed with PBS for 5 minutes x 3 times; 0.5 % goat serum was blocked at room temperature for 30 minutes; monoclonal mouse anti-human smooth muscle actin (MonoclonalMouse Anti-Human Smooth Muscle Actin Clone 1A4, 1:100, DakoCytomation Company), goat polyclonal anti-SM 22 α (Goat polyclonal to SM22 alpha , 1:100, American Abcam Company), mouse anti-smooth muscle myosin heavy chain monoclonal antibody (Mouse anti-smooth musclemyosin, heavy chain monoclonal anti ibody, 1:100, Canada CHEMICON Company), mouse monoclonal anti-Calponin antibody ( Mouse monoclonal to Calponin ab700, Abcam, UK) was incubated overnight at 4°C, rinsed with PBS, fluorescein isothiocyanate (FITC) mouse anti-rat IgG (1:100, Invitrogen, ...
Embodiment 3
[0081] Prepare vascular graft with the vascular smooth muscle cells obtained in Example 1
[0082] 1. Inoculation of vascular smooth muscle cells: within the second to third passage, the total number is 10×10 7 Add 2ml of DMEM culture medium to make cell suspension, inoculate the above cell suspension evenly on a piece of prefabricated PGA fiber, place at 37℃, 5% CO 2 In the incubator, add DMEM culture solution after 4 hours, place it in the incubator to continue culturing, and replace the culture solution every day.
[0083] 2. Construction of cell-PGA tubular complexes in vascular bioreactors.
[0084] (1) After 2 weeks of in vitro culture, the sheet-shaped cell-PGA complex was wrapped around a pre-sterilized silicone tube with an outer diameter of 6 mm, so that the cell-PGA complex formed a tubular structure.
[0085] (2) Set reactor parameters: flow rate: 200ml / min, after acting for 160ms, relax for 200ms, so that the pulsation reaches 167 times / min.
[0086] (3) In vit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com