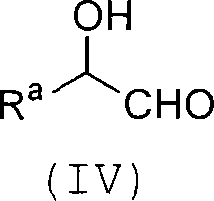
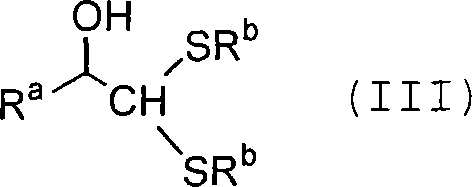
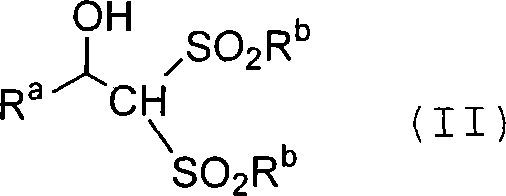Process for producing carbon-diminished aldose compound
A manufacturing method and compound technology, applied in the field of 5-deoxygenation, which is useful for synthesizing intermediates, can solve problems such as air pollution and deterioration of the operating environment, and achieve high industrial applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1: Production of L-rhamnose bis(dodecyl)thioacetal (XVI)
[0121] [chemical formula 17]
[0122]
[0123] Heat and dissolve 4.00g (22mmol) L-rhamnose (V) hydrate in 20mL dioxane, and add 8.89g (44mmol) n-dodecylmercaptan 4N hydrochloric acid little by little under stirring at room temperature / dioxane solution. After the dropwise addition, the reaction solution was transparent, and white crystals were deposited while the reaction was proceeding. Stir at room temperature for 12 hours, filter the precipitated white crystals, wash with ether and dry to obtain 9.00 g (yield 75%) of L-rhamnose bis(dodecyl) thiol (XVI) in the form of white crystals. ). This product can be directly put into the next reaction without purification, and colorless needle crystals can be obtained by recrystallization from dioxane.
[0124] Melting point: 111-112°C
[0125] IR spectrum (cm -1 ) 2917, 2850, 1467, 1064, 972, 894, 721, 613, 507, 422.
[0126] NMR spectrum (DMSO-D 6 ...
Embodiment 2
[0127] Example 2: 1,1-bis(dodecylsulfonyl)-1-mannose-2,3,4,5-tetrahydrohexane (XVII) manufacture
[0128] [chemical formula 18]
[0129]
[0130] 4.0 g (7.26 mmol) of the L-rhamnose bis(dodecyl)thioacetal (XVI) obtained in Example 1 above was dissolved by heating in 100 mL of acetic acid. To this solution, 40 mL of 30% aqueous hydrogen peroxide was added little by little under vigorous stirring, and after the addition was complete, stirring was continued at room temperature for 18 hours. The reaction solution from which a white precipitate precipitated was diluted with water, extracted with ethyl acetate, and the extract was dried and concentrated. Filter the resulting white precipitate, and obtain 4.02 g (yield 90%) of 1,1-bis(dodecylsulfonyl)-1-mannose-2,3,4,5-tetrahydrohexane (XVII) by drying . This product can be directly carried out in the next process, and colorless needle crystals can be obtained by recrystallization in acetic acid.
[0131] Melting point: 11...
Embodiment 3
[0134] Example 3: Production of 5-deoxy-L-arabinose (VIII)
[0135] [chemical formula 19]
[0136]
[0137] Dissolve 1.00 g (1.62 mmol) of 1,1-bis(dodecylsulfonyl)-1-mannose-2,3,4,5-tetrahydrohexane obtained in Example 2 above in 10 mL of dioxane (XVII), add 5 mL of 28% ammonia water under stirring. The mixture was stirred at room temperature for 1 hour, the precipitated white crystalline precipitate bis(dodecylsulfonyl)methane was removed by filtration, and the filtrate was concentrated. Water was added to the residue, extracted twice with chloroform, and the combined organic solvent was distilled off to obtain 0.17 g (yield 80%) of 5-deoxy-L-arabinose (VIII) as a pale yellow oil.
[0138] IR spectrum (cm -1 ): 3350, 1650, 1560, 1450, 1410, 1380, 1310, 1125, 1060, 1030, 980, 835.
[0139] NMR spectrum (D 2 O / ppm): 5.10 (1H, d), 5.07 (1H, d), 3.99 (1H, m), 3.90 (1H, m), 3.58 (1H, m), 1.63 (3H, d).
[0140] Each of the above-mentioned Examples 1 to 3 did not generate t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com