Probiotics and prebiotics for preventing and treating foetid faeces and foetid faeces poisoning syndrome and the composition preparations thereof
A technology of probiotics and prebiotics, which can be used in drug combinations, non-central analgesics, carbohydrate active ingredients, etc., can solve problems such as not many and insufficient awareness of harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Bacillus coagulans and Clostridium butyricum live bacteria are on the impact of ammonia and amine content in mouse feces
[0033] Method: 50 ICR purebred mice were divided into 5 groups, 10 mice in each group, and 10 live bacteria of Bacillus coagulans and Clostridium butyricum were used respectively. 9 cfu, 10 8 cfu / ml and physiological sodium chloride solution, 0.5ml / only, ig, once a day for a total of 21 days. The day before the end of the experiment, divide the mice into cages, one in each cage, take an equal amount of feces from each mouse, soak them in normal saline, then centrifuge to get the supernatant, and measure the amine value (μg / g) in the feces by titration . After the mice were sacrificed, the cecum was weighed, cut open in saline, and the contents were mixed well, then centrifuged to obtain the supernatant, and the ammonia value (μg / g) in the contents was determined with a corresponding kit.
[0034] Results Both Bacillus coagulans and C...
Embodiment 2
[0041] Example 2 Effects of Bacillus coagulans Live Bacteria Tablets and Clostridium Butyricum Capsules on Indole and Skatole Content in Human Feces
[0042] Methods HP-6890 gas chromatograph was used to determine the content of indole and skatole in the feces of 16 volunteers with informed consent. That is, take 0.5g of fresh feces and accurately weigh it, put it in a 50ml Erlenmeyer flask, add 20ml of ethanol for ultrasonic extraction for 20 minutes, filter, add 3ml of internal standard solution (0.1% p-Isopropylphenol), dilute to a 25ml measuring bottle, and measure 4 μl of the solution was injected into a chromatographic column (HP-INNO WAX glass capillary column, 30m×0.53mm, filled with 17% Silicone SE-30) for analysis. The measurement conditions are: column temperature: 200°C; inlet temperature: 230°C, detector temperature: 260°C, carrier gas flow rate N 2 90m1 / min; H2 Flow: 58ml / min; air flow: 60ml / min.
[0043] 16 volunteers, 6 males and 10 females, aged 25-55 years,...
Embodiment 3
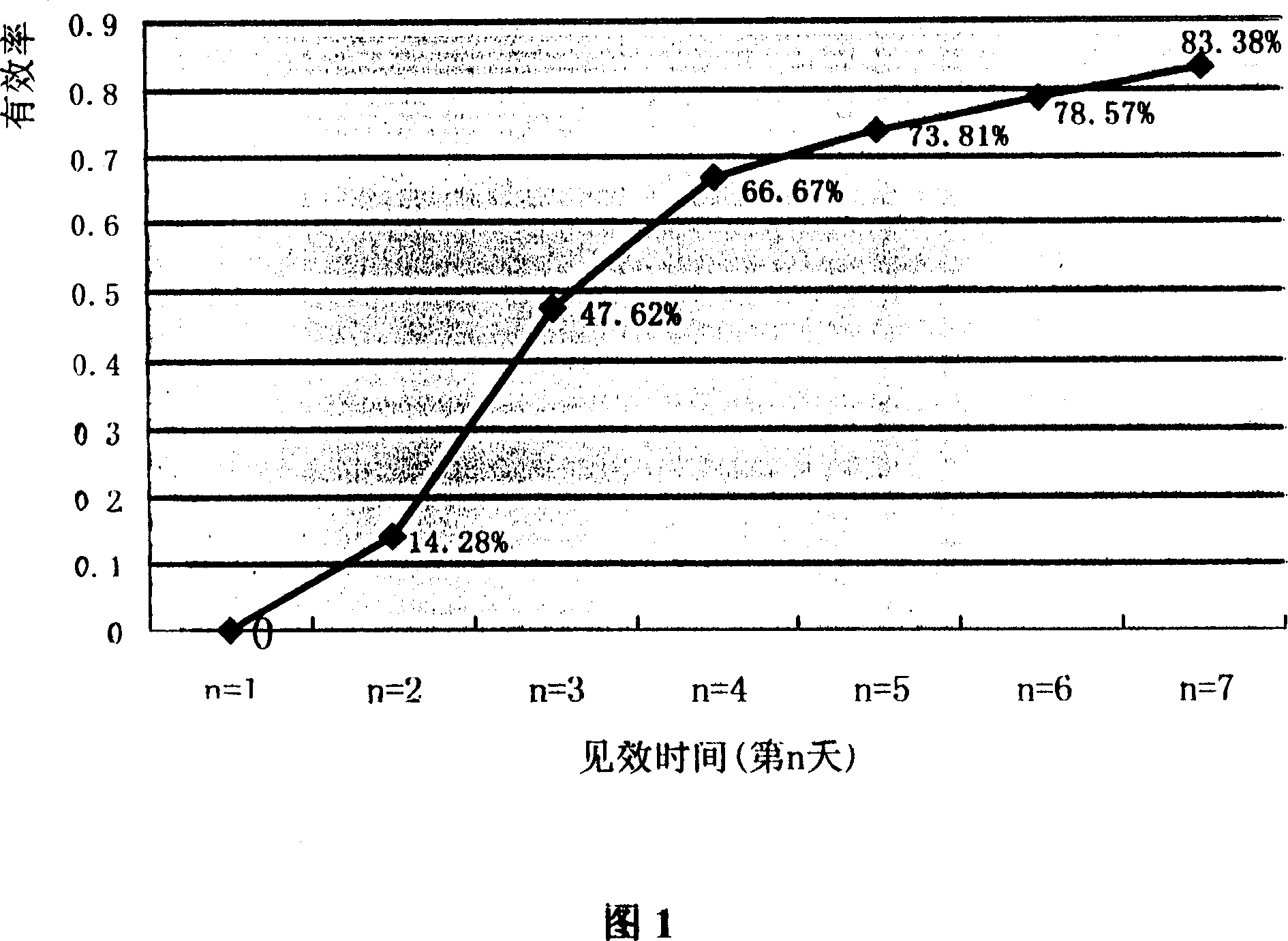
[0048] Example 3 Effects of Bacillus coagulans Live Bacteria Tablets on the Odor and Shape of Human Feces, etc.
[0049] Methods In the case of informed consent, 42 volunteers, 20 males and 22 females, aged 20-30 years old, maintained a normal diet and took live Bacillus coagulans tablets after meals, 3 tablets each time, 3 times a day, for 7 days in a row. sky. During the period of taking the medicine, observe the shape of the stool (normal-banana shape, strip shape, abnormal-chestnut shape, lumpy shape, muddy shape), hardness (normal-soft, abnormal-slightly hard, dry, hard), color ( Normal-yellow, light yellow-brown, yellow-colored, abnormal-dark brown, black, gray) and smell of stool (normal-smelling, abnormal-smell, very smelly, foul smell) and other conditions, Then count the test results.
[0050] Results The odor, shape, hardness, color and certain symptoms of the gastrointestinal tract of the volunteers after taking the medicine were significantly improved compared w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com